A novel alveolar epithelial cell sheet fabricated under feeder-free conditions for potential use in pulmonary regenerative therapy
- PMID: 35582208
- PMCID: PMC9073894
- DOI: 10.1016/j.reth.2022.01.005
A novel alveolar epithelial cell sheet fabricated under feeder-free conditions for potential use in pulmonary regenerative therapy
Abstract
Introduction: Lung transplantation is the only effective treatment option for many patients with irreversible pulmonary injury, and the demand for lung transplantation is increasing worldwide and expected to continue to outstrip the number of available donors. Regenerative therapy with alveolar epithelial cells (AECs) holds promise as an alternative option to organ transplantation. AECs are usually co-cultured with mouse-derived 3T3 feeder cells, but the use of xenogeneic tissues for regenerative therapy raises safety concerns. Fabrication of AEC sheets under feeder-free conditions would avoid these safety issues. We describe a novel feeder-free method of fabricating AEC sheets that may be suitable for pulmonary regenerative therapy.
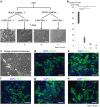
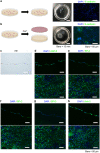
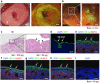
Methods: Lung tissues excised from male outbred rats or transgenic rats expressing green fluorescent protein (GFP) were finely minced and dissociated with elastase. The isolated AECs were cultured under four different feeder-free conditions according to whether a rho kinase (ROCK) inhibitor was included in the low-calcium medium (LCM) and whether the tissue culture dish was coated with recombinant laminin-511 E8 fragment (rLN511E8). The expanded cells were cultured on temperature-responsive dishes and subsequently harvested as AEC sheets. Engraftment of GFP-AEC sheets after their transplantation onto a partially resected region of the left lung was assessed in athymic rats.
Results: AECs proliferated and reached confluence when cultured in LCM containing a ROCK inhibitor on tissue culture dishes coated with rLN511E8. When both the ROCK inhibitor and rLN511E8-coated culture dish were used, the number of AECs obtained after 7 days of culture was significantly higher than that in the other three groups. Immunohistochemical analyses revealed that aquaporin-5, surfactant protein (SP)-A, SP-C, SP-D and Axin-2 were expressed by the cultured AECs. AEC sheets were harvested successfully from temperature-responsive culture dishes (by lowering the temperature) when the expanded AECs were cultured for 7 days in LCM + ROCK inhibitor and then for 3 days in LCM + ROCK inhibitor supplemented with 200 mg/L calcium chloride. The AEC sheets were firmly engrafted 7 days after transplantation onto the lung defect and expressed AEC marker proteins.
Conclusions: AEC sheets fabricated under feeder-free conditions retained the features of AECs after transplantation onto the lung in vivo. Further improvement of this technique may allow the bioengineering of alveolar-like tissue for use in pulmonary regenerative therapy.
Keywords: AEC, alveolar epithelial cell; AECI, type I alveolar epithelial cell; AECII, type II alveolar epithelial cell; AEpiCM, alveolar epithelial cell medium; AQP-5, aquaporin-5; Alveolar epithelial cell; Ca2+, ionized calcium; Cell sheet; FBS, fetal bovine serum; Feeder-free; GFP, green fluorescent protein; HBSS, Hanks' balanced salt solution; HE, hematoxylin and eosin; LCM, medium with a low ionized calcium concentration; PBS, phosphate-buffered saline; ROCK, rho kinase; Regenerative therapy; SP, surfactant protein; rLN511E8, recombinantly expressed laminin-511 E8 fragment.
© 2022 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Tatsuya Shimizu is a shareholder of CellSeed Inc. Tokyo Women's Medical University10.13039/501100010391Tokyo Women's Medical University received research funding from CellSeed Inc. The other authors have no competing interests to declare.
Figures

Similar articles
-
Explant culture of oral mucosal epithelial cells for fabricating transplantable epithelial cell sheet.Regen Ther. 2018 Dec 17;10:36-45. doi: 10.1016/j.reth.2018.10.006. eCollection 2019 Jun. Regen Ther. 2018. PMID: 30581895 Free PMC article.
-
Regulation of p53-mediated changes in the uPA-fibrinolytic system and in lung injury by loss of surfactant protein C expression in alveolar epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2017 Jun 1;312(6):L783-L796. doi: 10.1152/ajplung.00291.2016. Epub 2017 Apr 6. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28385810 Free PMC article.
-
Hyperoxia stimulates the transdifferentiation of type II alveolar epithelial cells in newborn rats.Am J Physiol Lung Cell Mol Physiol. 2015 May 1;308(9):L861-72. doi: 10.1152/ajplung.00099.2014. Epub 2015 Feb 13. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25681436
-
Breakdown of Epithelial Barrier Integrity and Overdrive Activation of Alveolar Epithelial Cells in the Pathogenesis of Acute Respiratory Distress Syndrome and Lung Fibrosis.Biomed Res Int. 2015;2015:573210. doi: 10.1155/2015/573210. Epub 2015 Oct 7. Biomed Res Int. 2015. PMID: 26523279 Free PMC article. Review.
-
Cryopreservation of Cell Sheets for Regenerative Therapy: Application of Vitrified Hydrogel Membranes.Gels. 2023 Apr 10;9(4):321. doi: 10.3390/gels9040321. Gels. 2023. PMID: 37102933 Free PMC article. Review.
Cited by
-
Tailoring cell sheets for biomedical applications.Smart Med. 2024 Feb 18;3(1):e20230038. doi: 10.1002/SMMD.20230038. eCollection 2024 Feb. Smart Med. 2024. PMID: 39188516 Free PMC article. Review.
-
Isogenic Transplantation of Hybrid Artificial Pleural Tissue Consisting of Rat Cells and Polyglycolic Acid Nanofiber Sheet Induces Restoration of Mesothelial Defects in Rat Model.Artif Organs. 2025 May;49(5):778-789. doi: 10.1111/aor.14947. Epub 2025 Jan 16. Artif Organs. 2025. PMID: 39817871 Free PMC article.
References
-
- Edgar L., Pu T., Porter B., Aziz J.M., La Pointe C., Asthana A., et al. Regenerative medicine, organ bioengineering and transplantation. Br J Surg. 2020;107:793–800. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

