Intracrine androgen biosynthesis and drug resistance
- PMID: 35582223
- PMCID: PMC8992556
- DOI: 10.20517/cdr.2020.60
Intracrine androgen biosynthesis and drug resistance
Abstract
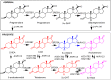
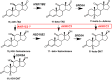
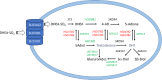
Castration-resistant prostate cancer is the lethal form of prostate cancer and most commonly remains dependent on androgen receptor (AR) signaling. Current therapies use AR signaling inhibitors (ARSI) exemplified by abiraterone acetate, a P450c17 inhibitor, and enzalutamide, a potent AR antagonist. However, drug resistance to these agents occurs within 12-18 months and they only prolong overall survival by 3-4 months. Multiple mechanisms can contribute to ARSI drug resistance. These mechanisms can include but are not limited to germline mutations in the AR, post-transcriptional alterations in AR structure, and adaptive expression of genes involved in the intracrine biosynthesis and metabolism of androgens within the tumor. This review focuses on intracrine androgen biosynthesis, how this can contribute to ARSI drug resistance, and therapeutic strategies that can be used to surmount these resistance mechanisms.
Keywords: Prostate cancer; abiraterone acetate; aldo-keto reductase 1C3; androgen biosynthesis; enzalutamide.
© The Author(s) 2020.
Conflict of interest statement
Penning TM is Founder Penzymes, LLC, he receives sponsored research funding from Forendo and serves on the Expert Panel for Research Institute for Fragrance Materials. Plymate S is president of ProsTech, Inc. All other authors declared that there are no conflicts of interest.
Figures

References
-
- Huggins CB, Hodges CV. Studies on prostatic cancer 1. Effect of castration, estrogen and androgen injection on serum phosphatases in metatstatic carcinoma of the prostate. Cancer Res. 1941;1:293–397.
-
- Huggins CB. Two principles in endocrine therapy of cancers: Hormone deprival and hormone interference. Cancer Res. 1965;25:1163–7. - PubMed
-
- Seely JH. Phase III studies on prostatic cancer with leuprolide acetate. J Androl. 1987;8:S23–6. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
