Novel approaches to combat chemoresistance against glioblastomas
- PMID: 35582224
- PMCID: PMC8992560
- DOI: 10.20517/cdr.2020.38
Novel approaches to combat chemoresistance against glioblastomas
Abstract
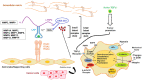
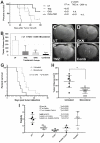
The poor prognosis of glioblastoma multiforme (GBM) patients is in part due to resistance to current standard-of-care treatments including chemotherapy [predominantly temozolomide (TMZ; Temodar)], radiation therapy and an anti-angiogenic therapy [an antibody against the vascular endothelial growth factor (bevacizumab; Avastin)], resulting in recurrent tumors. Several recurrent GBM tumors are commonly resistant to either TMZ, radiation or bevacizumab, which contributes to the low survival rate for GBM patients. This review will focus on novel targets and therapeutic approaches that are currently being considered to combat GBM chemoresistance. One of these therapeutic options is a small molecule called OKlahoma Nitrone 007 (OKN-007), which was discovered to inhibit the transforming growth factor β1 pathway, reduce TMZ-resistance and enhance TMZ-sensitivity. OKN-007 is currently an investigational new drug in clinical trials for both newly-diagnosed and recurrent GBM patients. Another novel target is ELTD1 (epidermal growth factor, latrophilin and seven transmembrane domain-containing protein 1; alternatively known as ADGRL4, Adhesion G protein-coupled receptor L4), which we used a monoclonal antibody against, where a therapy against it was found to inhibit Notch 1 in a pre-clinical GBM xenograft model. Notch 1 is known to be associated with chemoresistance in GBM. Other potential therapeutic targets to combat GBM chemoresistance include the phosphoinositide 3-kinase pathway, nuclear factor-κB, the hepatocyte/scatter factor (c-MET), the epidermal growth factor receptor, and the tumor microenvironment.
Keywords: ELTD1; Glioblastoma; OKlahoma Nitrone 007; magnetic resonance imaging; pre-clinical models; transforming growth factor-β1.
© The Author(s) 2020.
Conflict of interest statement
Towner RA holds patents for the use of OKN-007 and antibody therapy against ELTD1. All other co-authors declared that there are no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
