Resistance to second generation antiandrogens in prostate cancer: pathways and mechanisms
- PMID: 35582225
- PMCID: PMC8992566
- DOI: 10.20517/cdr.2020.45
Resistance to second generation antiandrogens in prostate cancer: pathways and mechanisms
Abstract
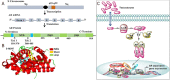
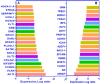
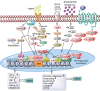
Androgen deprivation therapy targeting the androgens/androgen receptor (AR) signaling continues to be the mainstay treatment of advanced-stage prostate cancer. The use of second-generation antiandrogens, such as abiraterone acetate and enzalutamide, has improved the survival of prostate cancer patients; however, a majority of these patients progress to castration-resistant prostate cancer (CRPC). The mechanisms of resistance to antiandrogen treatments are complex, including specific mutations, alternative splicing, and amplification of oncogenic proteins resulting in dysregulation of various signaling pathways. In this review, we focus on the major mechanisms of acquired resistance to second generation antiandrogens, including AR-dependent and AR-independent resistance mechanisms as well as other resistance mechanisms leading to CRPC emergence. Evolving knowledge of resistance mechanisms to AR targeted treatments will lead to additional research on designing more effective therapies for advanced-stage prostate cancer.
Keywords: Prostate cancer; androgen receptor; castration resistance prostate cancer; second-generation antiandrogens.
© The Author(s) 2020.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures

References
-
- Leslie SW, Soon-Sutton TL, Sajjad H, Siref LE. Prostate cancer. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
