Effect of a specific Escherichia coli Nissle 1917 strain on minimal/mild hepatic encephalopathy treatment
- PMID: 35582294
- PMCID: PMC9055191
- DOI: 10.4254/wjh.v14.i3.634
Effect of a specific Escherichia coli Nissle 1917 strain on minimal/mild hepatic encephalopathy treatment
Abstract
Background: Hepatic encephalopathy (HE) can be considered a result of dysregulated gut-liver-brain axis function, where cognitive impairment can be reversed or prevented by the beneficial effects induced by "gut-centric" therapies, such as the administration of nonabsorbable disaccharides, nonabsorbable antibiotics, probiotics and prebiotics.
Aim: To assess the short-term efficacy and safety of the probiotic Escherichia coli Nissle (EcN) 1917 strain compared to lactulose and rifaximin in patients with minimal/mild HE.
Methods: From January 2017 to March 2020, a total of 45 patients with HE were enrolled in this prospective, single-centre, open-label, randomized study. Participants were randomly assigned at a ratio of 1:1:1 to one of the treatment groups: The EcN group (n = 15), lactulose group (n = 15) or rifaximin group (n = 15) for a 1 mo intervention period. The main primary outcomes of the study were changes in serum ammonia and Stroop test score. The secondary outcomes were markers of a chronic systemic inflammatory response (ІL-6, ІL-8, and IFN-γ) and bacteriology of the stool flora evaluated by specialized nonculture techniques after a 1 mo intervention period.
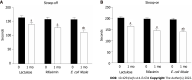
Results: Patients who were given rifaximin or EcN showed a more significant reduction in serum ammonia and normalization of Bifidobacteria and Lactobacilli abundance compared to the lactulose group. However, the most pronounced restoration of the symbiotic microflora was associated with EcN administration and characterized by the absence of E. coli with altered properties and pathogenic enterobacteria in patient faeces. In the primary outcome analysis, improvements in the Stroop test parameters in all intervention groups were observed. Moreover, EcN-treated patients performed 15% faster on the Stroop test than the lactulose group patients (P = 0.017). Both EcN and rifaximin produced similar significant reductions in the proinflammatory cytokines INF-γ, IL-6 and IL-8. EcN was more efficient than lactulose in reducing proinflammatory cytokine levels.
Conclusion: The use of the probiotic EcN strain was safe and quite efficient for HE treatment. The probiotic reduced the ammonia content and the level of serum proinflammatory cytokines, normalized the gut microbiota composition and improved the cognitive function of patients with HE. The application of the EcN strain was more effective than lactulose treatment.
Keywords: Chronic liver disease; Cognitive functions; E. coli Nissle 1917; Gut microbiota; Hepatic encephalopathy; Lactulose; Rifaximin; Stroop test; cirrhosis.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors declare no potential conflicting interests related to this paper.
Figures



References
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology . 2014;60:715–735. - PubMed
-
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther . 2007;25 Suppl 1:3–9. - PubMed
-
- de Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJC, Kramer M, Tjwa ETTL, Mostafavi N, Dijkgraaf MGW, van Delden OM, Beuers UHW, Coenraad MJ, Takkenberg RB. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial) BMJ Open Gastroenterol . 2020;7 - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

