Protective autophagy by thymidine causes resistance to rapamycin in colorectal cancer cells in vitro
- PMID: 35582304
- PMCID: PMC9094079
- DOI: 10.20517/cdr.2021.21
Protective autophagy by thymidine causes resistance to rapamycin in colorectal cancer cells in vitro
Abstract
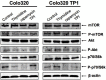
Aim: Thynidine phosphorylase (TP) acts as a proangiogenic growth factor which may regulate mammalian Target of Rapamycin (mTOR). We investigated whether the TP substrate thymidine and overexpression of TP affected mTOR signaling by comparing Colo320 (TP deficient) cells and its TP-transfected variant (Colo320TP1). Methods: Drug resistance was assessed with the sulforhodamine B assay, protein expression with Western blotting, cell cycle distribution and cell death with Fluorescence-activated cell sorting analysis, and autophagy with immunofluorescence. Results: Colo320 and Colo320TP1 cells had comparable levels of sensitivity to the mTOR inhibitor rapamycin. Thymidine treatment led to 13- and 50-fold resistance to rapamycin in Colo320 and Colo320TP1 cells, respectively. In Colo320TP1 cells, the thymidine phosphorylase inhibitor (TPI) reversed the thymidine induced resistance to rapamycin, but not in Colo320 cells, indicating a role for TP in the protection. Thymidine increased p70/S6k-phosphorylation (downstream of mTOR) in Colo320TP1, but it was not affected in Colo320. As a mechanism behind resistance, we studied the levels of autophagy and found that, in Colo320TP1 cells, autophagy was highly induced by thymidine-rapamycin, which was decreased by TPI. In addition, the autophagy inhibitor 3-methyl-adenine completely inhibited autophagy and its protection. Conclusion: Rapamycin resistance in TP-expressing cancer cells may therefore be related to thymidine-mediated autophagy activation.
Keywords: Thymidine phosphorylase; autophagy; mTOR; rapamycin; thymidine; thymidine phosphorylase inhibitor.
© The Author(s) 2021.
Conflict of interest statement
Both authors declared that there are no conflicts of interest.
Figures


Similar articles
-
Thymidine phoshorylase as a target for antiangiogenesis treatment.Nucleic Acids Symp Ser (Oxf). 2008;(52):629. doi: 10.1093/nass/nrn318. Nucleic Acids Symp Ser (Oxf). 2008. PMID: 18776537
-
The Hollow Fibre Assay as a model for in vivo pharmacodynamics of fluoropyrimidines in colon cancer cells.Br J Cancer. 2007 Jan 15;96(1):61-6. doi: 10.1038/sj.bjc.6603507. Epub 2006 Dec 19. Br J Cancer. 2007. PMID: 17179993 Free PMC article.
-
Activity and substrate specificity of pyrimidine phosphorylases and their role in fluoropyrimidine sensitivity in colon cancer cell lines.Int J Biochem Cell Biol. 2007;39(3):565-75. doi: 10.1016/j.biocel.2006.10.009. Epub 2006 Oct 21. Int J Biochem Cell Biol. 2007. PMID: 17098463
-
The role of platelet-derived endothelial cell growth factor/thymidine phosphorylase in tumor behavior.Nucleosides Nucleotides Nucleic Acids. 2008 Jun;27(6):681-91. doi: 10.1080/15257770802143988. Nucleosides Nucleotides Nucleic Acids. 2008. PMID: 18600526 Review.
-
Thymidine phosphorylase in cancer aggressiveness and chemoresistance.Pharmacol Res. 2018 Jun;132:15-20. doi: 10.1016/j.phrs.2018.03.019. Epub 2018 Mar 28. Pharmacol Res. 2018. PMID: 29604437 Review.
Cited by
-
Breast cancer cell resistance to hormonal and targeted therapeutics is correlated with the inactivation of the NR6A1 axis.Cancer Drug Resist. 2024 Nov 23;7:48. doi: 10.20517/cdr.2024.69. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39624078 Free PMC article.
-
Recent advances in tumor-targeting chemotherapy drugs.Cancer Drug Resist. 2021;4(4):885-887. doi: 10.20517/cdr.2021.86. Epub 2021 Sep 8. Cancer Drug Resist. 2021. PMID: 35309517 Free PMC article. No abstract available.
References
-
- Bruin M, Temmink OH, Hoekman K, Pinedo HM, Peters GJ. Role of platelet derived endothelial cell growth factor/thymidine phosphorylase in health and disease. Cancer Therapy. 2006;4:99–124.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
