Drug resistance in targeted cancer therapies with RAF inhibitors
- PMID: 35582307
- PMCID: PMC9094075
- DOI: 10.20517/cdr.2021.36
Drug resistance in targeted cancer therapies with RAF inhibitors
Abstract
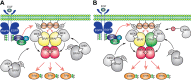
Hyperactive RAS/RAF/MEK/ERK signaling has a well-defined role in cancer biology. Targeting this pathway results in complete or partial regression of most cancers. In recent years, cancer genomic studies have revealed that genetic alterations that aberrantly activate the RAS/RAF/MEK/ERK signaling mainly occur on RAF or upstream, which motivated the extensive development of RAF inhibitors for cancer therapy. Currently, the first-generation RAF inhibitors have been approved for treating late-stage cancers with BRAF(V600E) mutations. Although these inhibitors have achieved promising outcomes in clinical treatments, their efficacy is abolished by quick-rising drug resistance. Moreover, cancers with hyperactive RAS exhibit intrinsic resistance to these drugs. To resolve these problems, the second-generation RAF inhibitors have been designed and are undergoing clinical evaluations. Here, we summarize the recent findings from mechanistic studies on RAF inhibitor resistance and discuss the critical issues in the development of next-generation RAF inhibitors with better therapeutic index, which may provide insights for improving targeted cancer therapy with RAF inhibitors.
Keywords: RAF inhibitors; RAF/KSR family kinase; RAS/RAF/MEK/ERK signaling; drug resistance; oncogenic mutation; regulatory spine; targeted therapy.
© The Author(s) 2021.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures

Similar articles
-
Targeting oncogenic Raf protein-serine/threonine kinases in human cancers.Pharmacol Res. 2018 Sep;135:239-258. doi: 10.1016/j.phrs.2018.08.013. Epub 2018 Aug 15. Pharmacol Res. 2018. PMID: 30118796 Review.
-
Complexity in KSR function revealed by Raf inhibitor and KSR structure studies.Small GTPases. 2011 Sep;2(5):276-281. doi: 10.4161/sgtp.2.5.17740. Epub 2011 Sep 1. Small GTPases. 2011. PMID: 22292131 Free PMC article.
-
Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy.Leukemia. 2011 Jul;25(7):1080-94. doi: 10.1038/leu.2011.66. Epub 2011 Apr 15. Leukemia. 2011. PMID: 21494257 Review.
-
Targeting RAS-RAF-MEK-ERK signaling pathway in human cancer: Current status in clinical trials.Genes Dis. 2022 May 20;10(1):76-88. doi: 10.1016/j.gendis.2022.05.006. eCollection 2023 Jan. Genes Dis. 2022. PMID: 37013062 Free PMC article. Review.
-
An oncogenic mutant of RHEB, RHEB Y35N, exhibits an altered interaction with BRAF resulting in cancer transformation.BMC Cancer. 2018 Jan 10;18(1):69. doi: 10.1186/s12885-017-3938-5. BMC Cancer. 2018. PMID: 29320991 Free PMC article.
Cited by
-
Hydroxytyrosol Reprograms the Tumor Microenvironment in 3D Melanoma Models by Suppressing ERBB Family and Kinase Pathways.Int J Mol Sci. 2025 Jul 20;26(14):6957. doi: 10.3390/ijms26146957. Int J Mol Sci. 2025. PMID: 40725203 Free PMC article.
-
Non-Small Cell Lung Cancer Targeted Therapy: Drugs and Mechanisms of Drug Resistance.Int J Mol Sci. 2022 Dec 1;23(23):15056. doi: 10.3390/ijms232315056. Int J Mol Sci. 2022. PMID: 36499382 Free PMC article. Review.
-
Histone deacetylases in the regulation of cell death and survival mechanisms in resistant BRAF-mutant cancers.Cancer Drug Resist. 2025 Jan 25;8:6. doi: 10.20517/cdr.2024.125. eCollection 2025. Cancer Drug Resist. 2025. PMID: 39935431 Free PMC article. Review.
-
ERBB2 as a prognostic biomarker correlates with immune infiltrates in papillary thyroid cancer.Front Genet. 2022 Nov 9;13:966365. doi: 10.3389/fgene.2022.966365. eCollection 2022. Front Genet. 2022. PMID: 36437939 Free PMC article.
-
First-in-human study of naporafenib (LXH254) with or without spartalizumab in adult patients with advanced solid tumors harboring MAPK signaling pathway alterations.Eur J Cancer. 2024 Jan;196:113458. doi: 10.1016/j.ejca.2023.113458. Epub 2023 Nov 21. Eur J Cancer. 2024. PMID: 38039779 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
