Cellular irinotecan resistance in colorectal cancer and overcoming irinotecan refractoriness through various combination trials including DNA methyltransferase inhibitors: a review
- PMID: 35582377
- PMCID: PMC8992440
- DOI: 10.20517/cdr.2021.82
Cellular irinotecan resistance in colorectal cancer and overcoming irinotecan refractoriness through various combination trials including DNA methyltransferase inhibitors: a review
Abstract
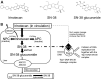
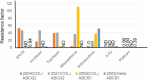
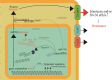
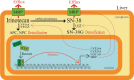
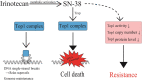
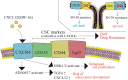
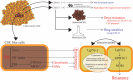
Treatment with pharmacological drugs for colorectal cancer (CRC) remains unsatisfactory. A major cause of failure in pharmacotherapy is the resistance of colon cancer cells to the drugs, creating an urgent issue. In this review, we summarize previous studies on the resistance of CRC cells to irinotecan and discuss possible reasons for refractoriness. Our review presents the following five major causes of irinotecan resistance in human CRC: (1) cellular irinotecan resistance is induced mainly through the increased expression of the drug efflux transporter, ABCG2; (2) cellular irinotecan resistance is also induced in association with a nuclear receptor, pregnane/steroid X receptor (PXR/SXR), which is enriched in the CYP3A4 gene enhancer region in CRC cells by exposing the cells to SN-38; (3) irinotecan-resistant cells possess either reduced DNA topoisomerase I (Top1) expression at both the mRNA and protein levels or Top1 missense mutations; (4) alterations in the tumor microenvironment lead to drug resistance through intercellular vesicle-mediated transmission of miRNAs; and (5) CRC stem cells are the most difficult targets to successfully treat CRC. In the clinical setting, CRC gradually develops resistance to initially effective irinotecan-based therapy. To solve this problem, several clinical trials, such as irinotecan plus cetuximab vs. cetuximab monotherapy, have been conducted. Another clinical trial on irinotecan plus guadecitabine, a DNA-methyltransferase inhibitor, has also been conducted.
Keywords: ABCG2; DNA topoisomerase I; Drug resistance; anti-cancer drugs; cancer stem cells; colorectal cancer; epigenetics; irinotecan.
© The Author(s) 2021.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures

Similar articles
-
MiR-3664-3p through suppressing ABCG2, CYP3A4, MCL1, and MLH1 increases the sensitivity of colorectal cancer cells to irinotecan.Heliyon. 2025 Jan 15;11(3):e41933. doi: 10.1016/j.heliyon.2025.e41933. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39931465 Free PMC article.
-
Establishment and Characterization of an Irinotecan-Resistant Human Colon Cancer Cell Line.Front Oncol. 2021 Feb 22;10:624954. doi: 10.3389/fonc.2020.624954. eCollection 2020. Front Oncol. 2021. PMID: 33692943 Free PMC article.
-
Pregnane X Receptor (PXR) expression in colorectal cancer cells restricts irinotecan chemosensitivity through enhanced SN-38 glucuronidation.Mol Cancer. 2010 Mar 2;9:46. doi: 10.1186/1476-4598-9-46. Mol Cancer. 2010. PMID: 20196838 Free PMC article.
-
Implications of ABCG2 Expression on Irinotecan Treatment of Colorectal Cancer Patients: A Review.Int J Mol Sci. 2017 Sep 7;18(9):1926. doi: 10.3390/ijms18091926. Int J Mol Sci. 2017. PMID: 28880238 Free PMC article. Review.
-
Cetuximab in the treatment of patients with colorectal cancer.Expert Opin Biol Ther. 2011 Jul;11(7):937-49. doi: 10.1517/14712598.2011.582464. Epub 2011 May 11. Expert Opin Biol Ther. 2011. PMID: 21557708 Review.
Cited by
-
Resistance to TOP-1 Inhibitors: Good Old Drugs Still Can Surprise Us.Int J Mol Sci. 2023 Apr 13;24(8):7233. doi: 10.3390/ijms24087233. Int J Mol Sci. 2023. PMID: 37108395 Free PMC article. Review.
-
Circulating miRNA Expression Profiles and Machine Learning Models in Association with Response to Irinotecan-Based Treatment in Metastatic Colorectal Cancer.Int J Mol Sci. 2022 Dec 20;24(1):46. doi: 10.3390/ijms24010046. Int J Mol Sci. 2022. PMID: 36613487 Free PMC article.
-
Efficacy and safety of sacituzumab govitecan Trop-2-targeted antibody-drug conjugate in solid tumors and UGT1A1*28 polymorphism: a systematic review and meta-analysis.BJC Rep. 2024 Nov 11;2(1):85. doi: 10.1038/s44276-024-00106-1. BJC Rep. 2024. PMID: 39528547 Free PMC article.
-
Gefitinib Inhibits Rifampicin-Induced CYP3A4 Gene Expression in Human Hepatocytes.ACS Omega. 2022 Sep 13;7(38):34034-34044. doi: 10.1021/acsomega.2c03270. eCollection 2022 Sep 27. ACS Omega. 2022. PMID: 36188260 Free PMC article.
-
Evaluation of CRC-Metastatic Hepatic Lesion Chemoembolization with Irinotecan-Loaded Microspheres, According to the Site of Embolization.J Pers Med. 2022 Mar 7;12(3):414. doi: 10.3390/jpm12030414. J Pers Med. 2022. PMID: 35330414 Free PMC article.
References
-
- Peters GJ. Drug resistance in colorectal cancer: general aspects. Drug resistance in colorectal cancer: molecular mechanisms and therapeutic strategies. Elsevier; 2020. p. 1-33. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
