Using cfRNA as a tool to evaluate clinical treatment outcomes in patients with metastatic lung cancers and other tumors
- PMID: 35582387
- PMCID: PMC8992448
- DOI: 10.20517/cdr.2021.78
Using cfRNA as a tool to evaluate clinical treatment outcomes in patients with metastatic lung cancers and other tumors
Abstract
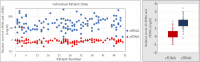
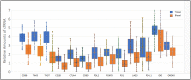
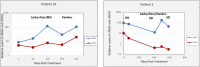
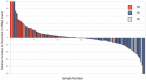
Aim: We report an exploratory analysis of cfRNA as a biomarker to monitor clinical responses in non-small cell lung cancer (NSCLC), breast cancer, and colorectal cancer (CRC). An analysis of cfRNA as a method for measuring PD-L1 expression with comparison to clinical responses was also performed in the NSCLC cohort. Methods: Blood samples were collected from 127 patients with metastatic disease that were undergoing therapy, 52 with NSCLC, 50 with breast cancer, and 25 with CRC. cfRNA was purified from fractionated plasma, and following reverse transcription (RT), total cfRNA and gene expression of PD-L1were analyzed by real-time polymerase chain reaction (qPCR) using beta-actin expression as a surrogate for relative amounts of cfDNA and cfRNA. For the concordance study of liquid biopsies and tissue biopsies, the isolated RNA was analyzed by RNAseq for the expressions of 13 genes. We had to close the study early due to a lack of follow-up during the Covid-19 pandemic. Results: We collected a total of 373 blood samples. Mean cfRNA PCR signals after RT were about 50-fold higher than those of cfDNA. cfRNA was detected in all patients, while cfDNA was detected in 88% of them. A high concordance was found for the expression levels of 13 genes between blood and solid tumor tissue. Changes in cfRNA levels followed over the course of treatments were associated with response to therapy, increasing in progressive disease (PD) and falling when a partial response (PR) occurred. The expression of PD-L1 over time in patients treated with immunotherapy decreased with PR but increased with PD. Pre-treatment levels of PD-L1 were predictive of response in patients treated with immunotherapy. Conclusion: Changes in cfRNA correlate with clinical response to the therapy. Total cfRNA may be useful in predicting clinical outcomes. PD-L1 gene expression may provide a biomarker to predict response to PD-L1 inhibition.
Keywords: NSCLC; PD-L1; cfDNA; cfRNA; liquid biopsies.
© The Author(s) 2021.
Conflict of interest statement
Raez LE received research support from Nant Health. Danenberg K, Soon-Shiong P, Reddy S: Employment and stocks in Nant Health. Huang E: Employment and stocks in Burning Rock. Usher J: Employment at Nant Health. Sands J, Castrellon A, Mililllo A, Sumarriva D, Danenberg P, Ferraro P declared that there are no conflicts of interests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
