Radial porosity profiles: a new bone histological method for comparative developmental analysis of diametric limb bone growth
- PMID: 35582660
- PMCID: PMC9091851
- DOI: 10.1098/rsos.211893
Radial porosity profiles: a new bone histological method for comparative developmental analysis of diametric limb bone growth
Abstract
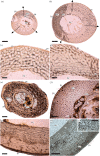
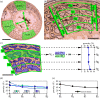
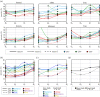
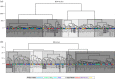
In fossil tetrapods, limb bone histology is considered the most reliable tool not only for inferring skeletal maturity-a crucial assessment in palaeobiological and evolutionary studies-but also for evaluating the growth dynamics within the ontogenetic window represented by the primary bone cortex. Due to its complex relationship with bone growth and functional maturation, primary cortical vascularity is an indispensable osteohistological character for reconstructing growth dynamics, especially in the context of various developmental strategies along the precocial-altricial spectrum. Using this concept as our working hypothesis, we developed a new quantitative osteohistological parameter, radial porosity profile (RPP), that captures relative cortical porosity changes in limb bones as trajectories. We built a proof-of-concept RPP dataset on extant birds, then added fossil paravian dinosaurs and performed a set of trajectory-grouping analyses to identify potential RPP categories and evaluate them in the context of our ontogeny-developmental strategy working hypothesis. We found that RPPs, indeed, reflect important developmental features within and across elements, specimens and taxa, supporting their analytical power. Our RPPs also revealed unexpected potential osteohistological correlates of growth and functional development of limb bones. The diverse potential applications of RPPs open up new research directions in the evolution of locomotor ontogeny.
Keywords: birds; growth and functional maturity; ontogeny; precocial–altricial development; quantitative bone histology; radial porosity profile.
© 2022 The Authors.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
-
- Chinsamy A. 1997. Assessing the biology of fossil vertebrates through bone histology. Palaeont. Afr. 33, 29-35.
-
- De Ricqlès A, Castanet J, Francillon-Vieillot H. 2004. The ‘message’ of bone tissue in paleoherpetology. Ital. J. Zool. 71, 3-12. (10.1080/11250000409356599) - DOI
-
- Varricchio DJ. 2010. A distinct dinosaur life history? Hist. Biol. 23, 91-107. (10.1080/08912963.2010.500379) - DOI
Associated data
LinkOut - more resources
Full Text Sources

