Roles of transient receptor potential channel 6 in glucose-induced cardiomyocyte injury
- PMID: 35582666
- PMCID: PMC9052005
- DOI: 10.4239/wjd.v13.i4.338
Roles of transient receptor potential channel 6 in glucose-induced cardiomyocyte injury
Abstract
Background: Diabetic cardiomyopathy (DCM) is a serious complication of end-stage diabetes that presents symptoms such as cardiac hypertrophy and heart failure. The transient receptor potential channel 6 (TRPC6) protein is a very important selective calcium channel that is closely related to the development of various cardiomyopathies.
Aim: To explore whether TRPC6 affects cardiomyocyte apoptosis and proliferation inhibition in DCM.
Methods: We compared cardiac function and myocardial pathological changes in wild-type mice and mice injected with streptozotocin (STZ), in addition to comparing the expression of TRPC6 and P-calmodulin-dependent protein kinase II (P-CaMKII) in them. At the same time, we treated H9C2 cardiomyocytes with high glucose and then evaluated the effects of addition of SAR, a TRPC6 inhibitor, and KN-93, a CaMKII inhibitor, to such H9C2 cells in a high-glucose environment.
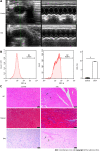
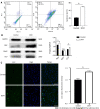
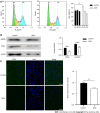
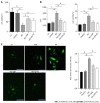
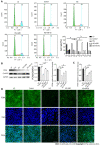
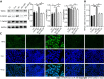
Results: We found that STZ-treated mice had DCM, decreased cardiac function, necrotic cardiomyocytes, and limited proliferation. Western blot and immunofluorescence were used to detect the expression levels of various appropriate proteins in the myocardial tissue of mice and H9C2 cells. Compared to those in the control group, the expression levels of the apoptosis-related proteins cleaved caspase 3 and Bax were significantly higher in the experimental group, while the expression of the proliferation-related proteins proliferating cell nuclear antigen (PCNA) and CyclinD1 was significantly lower. In vivo and in vitro, the expression of TRPC6 and P-CaMKII increased in a high-glucose environment. However, addition of inhibitors to H9C2 cells in a high-glucose environment resulted in alleviation of both apoptosis and proliferation inhibition.
Conclusion: The inhibition of apoptosis and proliferation of cardiomyocytes in a high-glucose environment may be closely related to activation of the TRPC6/P-CaMKII pathway.
Keywords: Apoptosis; Diabetic cardiomyopathy; H9C2 cells; P-calmodulin dependent protein kinase II; Proliferation; Transient receptor potential channel 6.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There are no relevant financial or non-financial interests to disclose.
Figures


Similar articles
-
Puerarin Attenuates High-Glucose and High-Lipid-Induced Inflammatory Injury in H9c2 Cardiomyocytes via CAV3 Protein Upregulation.J Inflamm Res. 2023 Jun 29;16:2707-2718. doi: 10.2147/JIR.S408681. eCollection 2023. J Inflamm Res. 2023. PMID: 37404717 Free PMC article.
-
SIRT6‑specific inhibitor OSS‑128167 exacerbates diabetic cardiomyopathy by aggravating inflammation and oxidative stress.Mol Med Rep. 2021 May;23(5):367. doi: 10.3892/mmr.2021.12006. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760202 Free PMC article.
-
Danshensu protects against ischemia/reperfusion injury and inhibits the apoptosis of H9c2 cells by reducing the calcium overload through the p-JNK-NF-κB-TRPC6 pathway.Int J Mol Med. 2016 Jan;37(1):258-66. doi: 10.3892/ijmm.2015.2419. Epub 2015 Nov 25. Int J Mol Med. 2016. PMID: 26718129
-
Activin receptor-like kinase 7 mediates high glucose-induced H9c2 cardiomyoblast apoptosis through activation of Smad2/3.Int J Biochem Cell Biol. 2013 Sep;45(9):2027-35. doi: 10.1016/j.biocel.2013.06.018. Epub 2013 Jul 2. Int J Biochem Cell Biol. 2013. PMID: 23830891
-
Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway.J Ethnopharmacol. 2019 Jun 28;238:111857. doi: 10.1016/j.jep.2019.111857. Epub 2019 Apr 6. J Ethnopharmacol. 2019. PMID: 30959142
Cited by
-
Differential changes in cyclic adenosine 3'-5' monophosphate (cAMP) effectors and major Ca2+ handling proteins during diabetic cardiomyopathy.J Cell Mol Med. 2023 May;27(9):1277-1289. doi: 10.1111/jcmm.17733. Epub 2023 Mar 27. J Cell Mol Med. 2023. PMID: 36967707 Free PMC article.
References
-
- Wu X, Huang L, Zhou X, Liu J. Curcumin protects cardiomyopathy damage through inhibiting the production of reactive oxygen species in type 2 diabetic mice. Biochem Biophys Res Commun. 2020;530:15–21. - PubMed
-
- Ma C, Luo H, Liu B, Li F, Tschöpe C, Fa X. Long noncoding RNAs: A new player in the prevention and treatment of diabetic cardiomyopathy? Diabetes Metab Res Rev. 2018;34:e3056. - PubMed
-
- Jammal Addin MB, Young D, McCarrison S, Hunter L. Dilated cardiomyopathy in a national paediatric population. Eur J Pediatr. 2019;178:1229–1235. - PubMed
-
- Barison A, Grigoratos C, Todiere G, Aquaro GD. Myocardial interstitial remodelling in non-ischaemic dilated cardiomyopathy: insights from cardiovascular magnetic resonance. Heart Fail Rev. 2015;20:731–749. - PubMed
-
- Gasparini S, Fonfara S, Kitz S, Hetzel U, Kipar A. Canine Dilated Cardiomyopathy: Diffuse Remodeling, Focal Lesions, and the Involvement of Macrophages and New Vessel Formation. Vet Pathol. 2020;57:397–408. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

