Radiomics signature: A potential biomarker for β-arrestin1 phosphorylation prediction in hepatocellular carcinoma
- PMID: 35582676
- PMCID: PMC9048469
- DOI: 10.3748/wjg.v28.i14.1479
Radiomics signature: A potential biomarker for β-arrestin1 phosphorylation prediction in hepatocellular carcinoma
Abstract
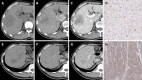
Background: The phosphorylation status of β-arrestin1 influences its function as a signal strongly related to sorafenib resistance. This retrospective study aimed to develop and validate radiomics-based models for predicting β-arrestin1 phosphorylation in hepatocellular carcinoma (HCC) using whole-lesion radiomics and visual imaging features on preoperative contrast-enhanced computed tomography (CT) images.
Aim: To develop and validate radiomics-based models for predicting β-arrestin1 phosphorylation in HCC using radiomics with contrast-enhanced CT.
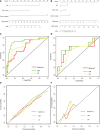
Methods: Ninety-nine HCC patients (training cohort: n = 69; validation cohort: n = 30) receiving systemic sorafenib treatment after surgery were enrolled in this retrospective study. Three-dimensional whole-lesion regions of interest were manually delineated along the tumor margins on portal venous CT images. Radiomics features were generated and selected to build a radiomics score using logistic regression analysis. Imaging features were evaluated by two radiologists independently. All these features were combined to establish clinico-radiological (CR) and clinico-radiological-radiomics (CRR) models by using multivariable logistic regression analysis. The diagnostic performance and clinical usefulness of the models were measured by receiver operating characteristic and decision curves, and the area under the curve (AUC) was determined. Their association with prognosis was evaluated using the Kaplan-Meier method.
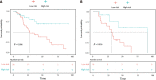
Results: Four radiomics features were selected to construct the radiomics score. In the multivariate analysis, alanine aminotransferase level, tumor size and tumor margin on portal venous phase images were found to be significant independent factors for predicting β-arrestin1 phosphorylation-positive HCC and were included in the CR model. The CRR model integrating the radiomics score with clinico-radiological risk factors showed better discriminative performance (AUC = 0.898, 95%CI, 0.820 to 0.977) than the CR model (AUC = 0.794, 95%CI, 0.686 to 0.901; P = 0.011), with increased clinical usefulness confirmed in both the training and validation cohorts using decision curve analysis. The risk of β-arrestin1 phosphorylation predicted by the CRR model was significantly associated with overall survival in the training and validation cohorts (log-rank test, P < 0.05).
Conclusion: The radiomics signature is a reliable tool for evaluating β-arrestin1 phosphorylation which has prognostic significance for HCC patients, providing the potential to better identify patients who would benefit from sorafenib treatment.
Keywords: Computed tomography; Hepatocellular carcinoma; Overall survival; Radiomics; Sorafenib resistance; β-Arrestin1 phosphorylation.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: We have no financial relationships to disclose.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin . 2021;71:209–249. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med . 2008;359:378–390. - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol . 2009;10:25–34. - PubMed
-
- Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, Lefkowitz RJ. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem . 1997;272:31051–31057. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

