The effect of the COVID-19 pandemic on severe asthma care in Europe: will care change for good?
- PMID: 35582679
- PMCID: PMC8994963
- DOI: 10.1183/23120541.00065-2022
The effect of the COVID-19 pandemic on severe asthma care in Europe: will care change for good?
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic has put pressure on healthcare services, forcing the reorganisation of traditional care pathways. We investigated how physicians taking care of severe asthma patients in Europe reorganised care, and how these changes affected patient satisfaction, asthma control and future care.
Methods: In this European-wide cross-sectional study, patient surveys were sent to patients with a physician-diagnosis of severe asthma, and physician surveys to severe asthma specialists between November 2020 and May 2021.
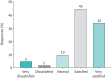
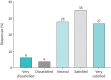
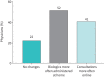
Results: 1101 patients and 268 physicians from 16 European countries contributed to the study. Common physician-reported changes in severe asthma care included use of video/phone consultations (46%), reduced availability of physicians (43%) and change to home-administered biologics (38%). Change to phone/video consultations was reported in 45% of patients, of whom 79% were satisfied or very satisfied with this change. Of 709 patients on biologics, 24% experienced changes in biologic care, of whom 92% were changed to home-administered biologics and of these 62% were satisfied or very satisfied with this change. Only 2% reported worsening asthma symptoms associated with changes in biologic care. Many physicians expect continued implementation of video/phone consultations (41%) and home administration of biologics (52%).
Conclusions: Change to video/phone consultations and home administration of biologics was common in severe asthma care during the COVID-19 pandemic and was associated with high satisfaction levels in most but not all cases. Many physicians expect these changes to continue in future severe asthma care, though satisfaction levels may change after the pandemic.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: A. Bourdin reports receiving grants or contracts outside the submitted work from AstraZeneca and Boeringher Ingelheim; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events as well as support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Chiesi and Amgen, outside the submitted work. Z. Csoma reports receiving honoraria for presentations from Astra/Zeneca, TEVA and Sanofi/Aventis (Hungary) outside the submitted work. E. Heffler reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Sanofi-Genzyme, Regeneron, Novartis, GSK, Circassia; Stallergenes-Greer and Nestlè Purina outside the submitted work. G.W. Canonica reports receiving consulting fees from AstraZeneca GSK, Novartis, Sanofi and Stallergenes Greer; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GSK, Novartis, Sanofi, Stallergenes Greer, Menarini, Chiesi and Mylan; participation on data safety monitoring or advisory boards for AstraZeneca, GSK, Novartis, Sanofi, Stallergenes Greer and Chiesi; all disclosures made outside the submitted work. G-J. Braunstahl reports grants or contracts from GSK, AstraZeneca and ALK Abello; consulting fees from GSK, Sanofi, ALK Abello, AstraZeneca and Novartis; payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from GSK, Sanofi, ALK Abello, AstraZeneca and Novartis; and is on the scientific board of the Dutch Lung Foundation, task force Asthma NVALT and editorial board NTVAAKI; all disclosures made outside the submitted work. S. van der Sar reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca and GSK; and support received from ALK for attending meetings and/or travel; all disclosures made outside the submitted work. N. Nenasheva reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca plc., Sanofi S.A., Teva Pharmaceuticals, Novartis International AG and Chiesi Farmaceutici S.p.A., outside the submitted work. A. Bossios reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AZ, GSK and Teva; support for attending meetings and/or travel received from Novartis; and participation on a data safety monitoring or advisory boards for AZ, GSK, Novartis, Teva and Sanofi; and is a member of the steering Committee of SHARP, Secretary of Assembly 5 (Airway diseases, asthma, COPD and chronic cough) of the European Respiratory Society and vice-chair of Nordic Severe Asthma Network; all disclosures made outside the submitted work. D. Aronsson reports receiving grants or contracts from ALK-Abello outside the submitted work. A. Egesten reports receiving honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, outside the submitted work. L. Ahlbeck reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca; and participation on data safety monitoring or advisory boards for AstraZeneca, Sanofi and GSK; all disclosures made outside the submitted work. S. Škrgat reports receiving honoraria for lectures and educational events supported by GSK, AstraZeneca, Sanofi, Chiesi, Pliva Teva and Medis; and participation on advisory boards of GSK, AstraZeneca, Chiesi and Sanofi; all disclosures made outside the submitted work. N. Edelbaher reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, Astra Zeneca, Chiesi, Pliva Teva, Krka, Novartis, Boehringer Ingelheim and Sanofi; and participation on advisory boards of GSK, Astra Zeneca, Chiesi, Novartis and Boehringer Ingelheim; all disclosures made outside the submitted work. J. Rüdiger reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from the Education of Swiss Emergency physicians; and participation on a data safety monitoring board or board for Boehringer Ingelheim; and is a member of the Board of the Swiss Society of Pneumology and President of the Thorax section of the Swiss Ultrasound Society; all disclosures made outside the submitted work. N. Pavlov reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, Novartis and OM Pharma; support for attending meetings and/or travel received from Boehringer Ingelheim; participation on data safety monitoring or advisory boards for AstraZeneca, GSK, Novartis and Sanofi; all disclosures made outside the submitted work. P. Gianella reports participation on an advisory board for Novartis about Xolair for nasal polyps, outside the submitted work. F. Charbonnier reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Sanofi, Novartis, Mundipharma, AstraZeneca and GSK; participation on data safety monitoring or advisory boards for Sanofi, Novartis, Mundipharma, AstraZeneca and GSK; all disclosures made outside the submitted work. R. Chaudhuri reports receiving grants or contracts from AstraZeneca; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, AstraZeneca, Teva, Chiesi, Sanofi and Novartis; support for attending meetings and/or travel received from Chiesi, Napp, Sanofi, Boehringer, GSK and AZ; and participation on data safety monitoring or advisory boards for GSK, AstraZeneca, Teva, Chiesi and Novartis; all disclosures made outside the submitted work. S.J. Smith is supported by the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement number 831434 for Taxonomy, Targets, Treatment, and Remission; the JU receives support from the European Union's Horizon 2020 research and innovation programme, and the European Federation of Pharmaceutical Industries and Associates; all disclosures made outside the submitted work. S. Doe reports receiving support for attending meetings and/or travel from GSK and Sanofi, outside the submitted work. L. Heaney reports grants or contracts from Medimmune, Novartis UK, Roche/Genentech Inc., GlaxoSmithKline, Amgen, Genentech/Hoffman la Roche, AstraZeneca, Medimmune, Aerocrine and Vitalograph; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for Novartis, Hoffman la Roche/Genentech Inc., Sanofi, GlaxoSmithKline, AstraZeneca, Teva and Circassia; support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Napp Pharmaceuticals; participation on data safety monitoring or advisory board for Novartis, Hoffman la Roche/Genentech Inc., Sanofi, Evelo Biosciences, GlaxoSmithKline, AstraZeneca, Teva, Theravance and Circassia; all disclosures made outside the submitted work. M. Masoli reports receiving grants or contracts from ERS SHARP to support the study “The burden of severe asthma on HRQoL across Europe”, outside the submitted work. P. Howarth is an employee of GSK. R. Djukanovic reports support for the present manuscript received from ERS, TEVA, GSK, Novartis, Sanofi and Chiesi; consulting fees from Synairgen for which the author is a co-founder and consultant and owns shares, outside the submitted work; and participation on a data safety monitoring or advisory board for Kymab (Cambridge), outside the submitted work. E. Bel reports support for the present manuscript from the ERS; grants or contracts received from GlaxoSmithKline and Teva, outside the submitted work; consulting fees received from AstraZeneca, GlaxoSmithKline, Sanofi, Sterna and Chiesi, outside the submitted work; honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Teva; participation on a data safety monitoring or advisory board for AstraZeneca, outside the submitted work; and leadership or fiduciary roles, unpaid, for SHARP-CRC and RAPSODI (Dutch registry), outside the submitted work. M. Hyland reports receiving grants or contracts from TEVA; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for GSK; all disclosures made outside the submitted work. The remaining authors have nothing to disclose.
Figures
References
-
- Global Initiative for Asthma (GINA) . Difficult-to-treat & severe asthma in adolescents and adult patients – Diagnosis and Management. V2.0. 2019. http://ginasthma.org/
Grants and funding
LinkOut - more resources
Full Text Sources
