Robotic Tissue Sampling for Safe Post-Mortem Biopsy in Infectious Corpses
- PMID: 35582701
- PMCID: PMC8956373
- DOI: 10.1109/TMRB.2022.3146440
Robotic Tissue Sampling for Safe Post-Mortem Biopsy in Infectious Corpses
Abstract
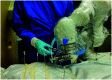
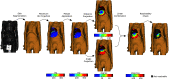
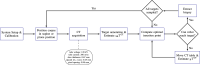
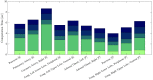
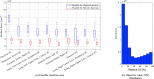
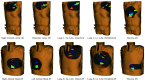
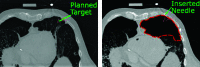
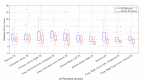
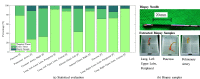
In pathology and legal medicine, the histopathological and microbiological analysis of tissue samples from infected deceased is a valuable information for developing treatment strategies during a pandemic such as COVID-19. However, a conventional autopsy carries the risk of disease transmission and may be rejected by relatives. We propose minimally invasive biopsy with robot assistance under CT guidance to minimize the risk of disease transmission during tissue sampling and to improve accuracy. A flexible robotic system for biopsy sampling is presented, which is applied to human corpses placed inside protective body bags. An automatic planning and decision system estimates optimal insertion point. Heat maps projected onto the segmented skin visualize the distance and angle of insertions and estimate the minimum cost of a puncture while avoiding bone collisions. Further, we test multiple insertion paths concerning feasibility and collisions. A custom end effector is designed for inserting needles and extracting tissue samples under robotic guidance. Our robotic post-mortem biopsy (RPMB) system is evaluated in a study during the COVID-19 pandemic on 20 corpses and 10 tissue targets, 5 of them being infected with SARS-CoV-2. The mean planning time including robot path planning is 5.72±167s. Mean needle placement accuracy is 7.19± 422mm.
Keywords: COVID-19; Collaborative robot; forensic medicine; medical robotics; path planning.
Figures




References
-
- Aghayev E., Thali M. J., Sonnenschein M., Jackowski C., Dirnhofer R., and Vock P., “Post-mortem tissue sampling using computed tomography guidance,” Forensic Sci. Int., vol. 166, nos. 2–3, pp. 199–203, 2007. - PubMed
-
- Lindner D.et al. , “Cardiac SARS-CoV-2 infection is associated with distinct transcriptomic changes within the heart,” Cardiovasc. Med., to be published.
LinkOut - more resources
Full Text Sources
Miscellaneous
