Hoxb5 reprogrammes murine multipotent blood progenitors into haematopoietic stem cell-like cells
- PMID: 35582777
- PMCID: PMC9201374
- DOI: 10.1111/cpr.13235
Hoxb5 reprogrammes murine multipotent blood progenitors into haematopoietic stem cell-like cells
Abstract
Objectives: The expression of transcription factor Hoxb5 specifically marks the functional haematopoietic stem cells (HSC) in mice. However, our recent work demonstrated that ectopic expression of Hoxb5 exerted little effect on HSC but could convert B-cell progenitors into functional T cells in vivo. Thus, cell type- and development stage-specific roles of Hoxb5 in haematopoietic hierarchy await more extensive exploration. In this study, we aim to investigate the effect of Hoxb5 expression in multipotent blood progenitor cells.
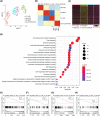
Materials and methods: A Mx1cre/RosaLSL-Hoxb5-EGFP/+ mouse model was used to evaluate the effect of Hoxb5 expression in blood multipotent progenitor cells (MPP). Golden standard serial transplantation experiments were used to test the long-term haematopoiesis potential of Hoxb5-expressing MPP. Single-cell RNA-seq analysis was used to characterize the gained molecular features of Hoxb5-expressing MPP and to compare with the global transcriptome features of natural adult HSC and fetal liver HSC (FL HSC).
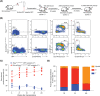
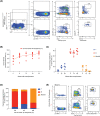
Results: Here, with a mouse strain engineered with conditional expression of Hoxb5, we unveiled that induced expression of Hoxb5 in MPP led to the generation of a de novo Sca1+ c-kit+ CD11b+ CD48+ (CD11b+ CD48+ SK) cell type, which can repopulate long-term multilineage haematopoiesis in serial transplantations. RNA-seq analysis showed that CD11b+ CD48+ SK cells exhibited acquired features of DNA replication and cell division.
Conclusions: Our current study uncovers that Hoxb5 can empower MPP with self-renewal ability and indicates an alternative approach for generating HSC-like cells in vivo from blood lineage cells.
© 2022 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no competing financial interests in relation to the work described.
Figures

Similar articles
-
Transcription factor Hoxb5 reveals the unidirectional hierarchy of hematopoietic stem cell pool.Stem Cell Res. 2024 Apr;76:103326. doi: 10.1016/j.scr.2024.103326. Epub 2024 Feb 1. Stem Cell Res. 2024. PMID: 38324932
-
Hoxb5 defines the heterogeneity of self-renewal capacity in the hematopoietic stem cell compartment.Biochem Biophys Res Commun. 2021 Feb 5;539:34-41. doi: 10.1016/j.bbrc.2020.12.077. Epub 2021 Jan 5. Biochem Biophys Res Commun. 2021. PMID: 33418191
-
NUP98-HOXA10hd fusion protein sustains multi-lineage haematopoiesis of lineage-committed progenitors in transplant setting.Cell Prolif. 2020 Sep;53(9):e12885. doi: 10.1111/cpr.12885. Epub 2020 Jul 29. Cell Prolif. 2020. PMID: 32725842 Free PMC article.
-
CPHEN-013: Comprehensive phenotyping of hematopoietic stem and progenitor cells in the human fetal liver.Cytometry A. 2022 Nov;101(11):903-908. doi: 10.1002/cyto.a.24540. Epub 2022 Mar 7. Cytometry A. 2022. PMID: 35253987 Review.
-
From haematopoietic stem cells to complex differentiation landscapes.Nature. 2018 Jan 24;553(7689):418-426. doi: 10.1038/nature25022. Nature. 2018. PMID: 29364285 Free PMC article. Review.
Cited by
-
Chromosomal Aberration t(14;17)(q32;q21) Simultaneously Activates HOXB5 and miR10a in Triple-Hit B-Cell Lymphoma.Biomedicines. 2023 Jun 19;11(6):1758. doi: 10.3390/biomedicines11061758. Biomedicines. 2023. PMID: 37371852 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

