Randomized clinical trial: Direct-acting antivirals as treatment for hepatitis C in people who inject drugs: Delivered in needle and syringe programs via directly observed therapy versus fortnightly collection
- PMID: 35582875
- PMCID: PMC9544056
- DOI: 10.1111/jvh.13701
Randomized clinical trial: Direct-acting antivirals as treatment for hepatitis C in people who inject drugs: Delivered in needle and syringe programs via directly observed therapy versus fortnightly collection
Abstract
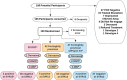
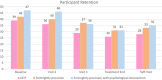
Hepatitis C virus (HCV) treatment in people who inject drugs (PWID) is delivered within settings frequented by PWID, such as needle and syringe programs (NSP). The optimal direct-acting antiviral (DAA) dispensing regimen among NSP clients is unknown. This study compared cures (Sustained virologic response 12 weeks post-treatment, [SVR12 ]) across three dispensing schedules to establish non-inferiority of fortnightly dispensing versus directly observed therapy. The ADVANCE HCV study was a randomized, unblinded trial, recruiting PWID attending NSP in Tayside, Scotland, between January 2018 and November 2019. HCV-positive participants were randomized to receive DAAs via directly observed therapy, fortnightly provision or fortnightly provision with psychological intervention. A modified intention to treat analysis was used to identify differences in cures between the three treatment regimes. The study was registered with clinicaltrials.gov; NCT03236506. A total of 110 participants completed the study. 33 participants received directly observed therapy, with 90.91% SVR12 ; 37 received fortnightly provision, with 86.49% SVR12 and 40 received fortnightly provision and psychological intervention at treatment initiation, with 92.50% SVR12 . Analysis showed no significant difference in SVR12 (p = 0.67). This study did not demonstrate a statistically significant difference in cure rate between groups. This provides evidence of the non-inferiority of fortnightly dispensing of direct-acting antivirals (DAAs) compared to directly observed therapy among PWID. It suggests that tight control of adherence through directly observed therapy dispensing of DAAs among this population offers no therapeutic advantage. Therefore, less restrictive dispensing patterns can be used, tailored to patient convenience.
Keywords: direct-acting antivirals; hepatitis c; needle and syringe programs; randomized controlled trial.
© 2022 The Authors. Journal of Viral Hepatitis published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Randomised controlled trial conducted in injecting equipment provision sites to compare the effectiveness of different hepatitis C treatment regimens in people who inject drugs: A Direct obserVed therApy versus fortNightly CollEction study for HCV treatment-ADVANCE HCV protocol study.BMJ Open. 2019 Aug 8;9(8):e029516. doi: 10.1136/bmjopen-2019-029516. BMJ Open. 2019. PMID: 31399460 Free PMC article.
-
Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy.J Hepatol. 2019 Jul;71(1):45-51. doi: 10.1016/j.jhep.2019.02.018. Epub 2019 Mar 8. J Hepatol. 2019. PMID: 30853642
-
Reduction in the population prevalence of hepatitis C virus viraemia among people who inject drugs associated with scale-up of direct-acting anti-viral therapy in community drug services: real-world data.Addiction. 2021 Oct;116(10):2893-2907. doi: 10.1111/add.15459. Epub 2021 May 5. Addiction. 2021. PMID: 33651446
-
Efficacy of Direct-acting Antivirals for Chronic Hepatitis C Virus Infection in People Who Inject Drugs or Receive Opioid Substitution Therapy: A Systematic Review and Meta-analysis.Clin Infect Dis. 2020 May 23;70(11):2355-2365. doi: 10.1093/cid/ciz696. Clin Infect Dis. 2020. PMID: 31513710
-
Hepatitis C Reinfection in People Who Inject Drugs in Resource-Limited Countries: A Systematic Review and Analysis.Int J Environ Res Public Health. 2020 Jul 9;17(14):4951. doi: 10.3390/ijerph17144951. Int J Environ Res Public Health. 2020. PMID: 32659974 Free PMC article.
Cited by
-
Laying the foundations for hepatitis C elimination: evaluating the development and contribution of community care pathways to diagnostic efforts.BMC Public Health. 2023 Jan 7;23(1):54. doi: 10.1186/s12889-022-14911-1. BMC Public Health. 2023. PMID: 36611156 Free PMC article. Review.
-
Predictors of hepatitis C cure among people who inject drugs treated with directly observed therapy supported by peer case managers in Kenya.Int J Drug Policy. 2023 Mar;113:103959. doi: 10.1016/j.drugpo.2023.103959. Epub 2023 Feb 7. Int J Drug Policy. 2023. PMID: 36758335 Free PMC article.
-
Five-year follow-up of sustained virological response with hepatitis C infection after direct-acting antiviral therapy: A single-center retrospective study.Medicine (Baltimore). 2024 Feb 16;103(7):e37212. doi: 10.1097/MD.0000000000037212. Medicine (Baltimore). 2024. PMID: 38363923 Free PMC article.
-
Prevention of Viral Hepatitis and HIV Infection among People Who Inject Drugs: A Systematic Review and Meta-Analysis.Viruses. 2024 Jan 18;16(1):142. doi: 10.3390/v16010142. Viruses. 2024. PMID: 38257842 Free PMC article.
References
-
- World Health Organisation . Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections 2019: Accountability for the Global Health Sector Strategies, 2016–2021. World Health Organization; 2019.
-
- World Health Organisation . Combating Hepatitis B and C to Reach Elimination by 2030. 2016; Available from: https://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_...
-
- D'Ambrosio R, Degasperi E, Colombo M, Aghemo A. Direct‐acting antivirals: the endgame for hepatitis C? Curr Opin Virol. 2017;24:31‐37. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical