icaR and icaT are Ancient Chromosome Genes Encoding Substrates of the Type III Secretion Apparatus in Shigella flexneri
- PMID: 35582904
- PMCID: PMC9241512
- DOI: 10.1128/msphere.00115-22
icaR and icaT are Ancient Chromosome Genes Encoding Substrates of the Type III Secretion Apparatus in Shigella flexneri
Abstract
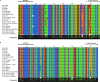
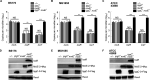
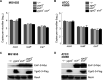
Shigella is an Escherichia coli pathovar that colonizes the cytosol of mucosal cells in the human large intestine. To do this, Shigella uses a Type III Secretion Apparatus (T3SA) to translocate several proteins into host cells. The T3SA and its substrates are encoded by genes of the virulence plasmid pINV or by chromosomal genes derived thereof. We recently discovered two chromosomal genes, which seem unrelated to pINV, although they are activated by MxiE and IpgC similarly to some of the canonical substrates of the T3SA. Here, we showed that the production of the corresponding proteins depended on the conservation of a MxiE box in their cognate promoters. Furthermore, both proteins were secreted by the T3SA in a chaperone-independent manner through the recognition of their respective amino-terminal secretion signal. Based on these observations, we named these new genes icaR and icaT, which stand for invasion chromosome antigen with homology for a transcriptional regulator and a transposase, respectively. icaR and icaT have orthologs in commensal and pathogenic E. coli strains belonging mainly to phylogroups A, B1, D and E. Finally, we demonstrated that icaR and icaT orthologs could be activated by the coproduction of IpgC and MxiE in strains MG1655 K-12 (phylogroup A) and O157:H7 ATCC 43888 (phylogroup E). In contrast, the coproduction of EivF and YgeG, which are homologs of MxiE and IpgC in the E. coli T3SS 2 (ETT2), failed to activate icaR and icaT. IMPORTANCEicaR and icaT are the latest members of the MxiE regulon discovered in the chromosome. The proteins IcaR and IcaT, albeit produced in small amounts, are nonetheless secreted by the T3SA comparably to canonical substrates. The high occurrence of icaR and icaT in phylogroups A, B1, D, and E coupled with their widespread absence in their B2 counterparts agree with the consensus E. coli phylogeny. The widespread conservation of the MxiE box among icaR and icaT orthologs supports the notion that both genes had already undergone coevolution with transcriptional activators ipgC and mxiE- harbored in pINV or a relative- in the last common ancestor of Shigella and of E. coli from phylogroups A, B1, D, and E. The possibility that icaR and icaT may contribute to Shigella pathogenesis cannot be excluded, although some of their characteristics suggest they are fossil genes.
Keywords: Escherichia coli; Shigella; phylogeny; transcription regulation; type III secretion system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Characterization of MxiE- and H-NS-Dependent Expression of ipaH7.8, ospC1, yccE, and yfdF in Shigella flexneri.mSphere. 2022 Dec 21;7(6):e0048522. doi: 10.1128/msphere.00485-22. Epub 2022 Nov 8. mSphere. 2022. PMID: 36346241 Free PMC article.
-
A Tale about Shigella: Evolution, Plasmid, and Virulence.Microorganisms. 2023 Jun 30;11(7):1709. doi: 10.3390/microorganisms11071709. Microorganisms. 2023. PMID: 37512882 Free PMC article. Review.
-
The AraC/XylS Protein MxiE and Its Coregulator IpgC Control a Negative Feedback Loop in the Transcriptional Cascade That Regulates Type III Secretion in Shigella flexneri.J Bacteriol. 2022 Jul 19;204(7):e0013722. doi: 10.1128/jb.00137-22. Epub 2022 Jun 15. J Bacteriol. 2022. PMID: 35703565 Free PMC article.
-
Characterization of the promoter, MxiE box and 5' UTR of genes controlled by the activity of the type III secretion apparatus in Shigella flexneri.PLoS One. 2012;7(3):e32862. doi: 10.1371/journal.pone.0032862. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22427898 Free PMC article.
-
Implications of Spatiotemporal Regulation of Shigella flexneri Type Three Secretion Activity on Effector Functions: Think Globally, Act Locally.Front Cell Infect Microbiol. 2016 Mar 9;6:28. doi: 10.3389/fcimb.2016.00028. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27014638 Free PMC article. Review.
Cited by
-
Characterization of MxiE- and H-NS-Dependent Expression of ipaH7.8, ospC1, yccE, and yfdF in Shigella flexneri.mSphere. 2022 Dec 21;7(6):e0048522. doi: 10.1128/msphere.00485-22. Epub 2022 Nov 8. mSphere. 2022. PMID: 36346241 Free PMC article.
-
A Tale about Shigella: Evolution, Plasmid, and Virulence.Microorganisms. 2023 Jun 30;11(7):1709. doi: 10.3390/microorganisms11071709. Microorganisms. 2023. PMID: 37512882 Free PMC article. Review.
-
The promiscuous biotin ligase TurboID reveals the proxisome of the T3SS chaperone IpgC in Shigella flexneri.mSphere. 2024 Nov 21;9(11):e0055324. doi: 10.1128/msphere.00553-24. Epub 2024 Oct 31. mSphere. 2024. PMID: 39480076 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

