Signaling pathways involved in NMDA-induced suppression of M-channels in corticotropin-releasing hormone neurons in central amygdala
- PMID: 35583089
- PMCID: PMC10210974
- DOI: 10.1111/jnc.15647
Signaling pathways involved in NMDA-induced suppression of M-channels in corticotropin-releasing hormone neurons in central amygdala
Abstract
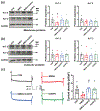
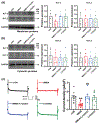
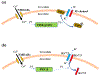
Glutamate N-methyl-d-aspartate (NMDA) receptors (NMDARs) and Kv7/M channels are importantly involved in regulating neuronal activity involved in various physiological and pathological functions. Corticotropin-releasing hormone (CRH)-expressing neurons in the central nucleus of the amygdala (CeA) critically mediate autonomic response during stress. However, the interaction between NMDA receptors and Kv7/M channels in the CRHCeA neurons remains unclear. In this study, we identified rat CRHCeA neurons through the expression of an AAV viral vector-mediated enhanced green fluorescent protein (eGFP) driven by the rat CRH promoter. M-currents carried by Kv7/M channels were recorded using the whole-cell patch-clamp approach in eGFP-tagged CRHCeA neurons in brain slices. Acute exposure to NMDA significantly reduced M-currents recorded from the CRHCeA neurons. NMDA-induced suppression of M-currents was eliminated by chelating intracellular Ca2+ , supplying phosphatidylinositol 4,5-bisphosphate (PIP2) intracellularly, or blocking phosphoinositide3-kinase (PI3K). In contrast, inhibiting protein kinase C (PKC) or calmodulin did not alter NMDA-induced suppression of M-currents. Sustained exposure of NMDA decreased Kv7.3 membrane protein levels and suppressed M-currents, while the Kv7.2 expression levels remained unaltered. Pre-treatment of brain slices with PKC inhibitors alleviated the decreases in Kv7.3 and reduction of M-currents in CRHCeA neurons induced by NMDA. PKC inhibitors did not alter Kv7.2 and Kv7.3 membrane protein levels and M-currents in CRHCeA neurons. These data suggest that transient activation of NMDARs suppresses M-currents through the Ca2+ -dependent PI3K-PIP2 signaling pathway. In contrast, sustained activation of NMDARs reduces Kv7.3 protein expression and suppresses M-currents through a PKC-dependent pathway.
Keywords: Corticotropin-releasing hormone (CRH)-expressing neurons; Kv7/M channel; NMDA receptor; amygdala; protein kinase C.
© 2022 International Society for Neurochemistry.
Conflict of interest statement
Conflict(s) of Interest/Disclosure(s)
The authors declare no competing financial interests.
Figures


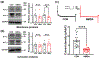

Similar articles
-
Corticotropin-releasing hormone neurons in the central nucleus of amygdala are required for chronic stress-induced hypertension.Cardiovasc Res. 2023 Jul 6;119(8):1751-1762. doi: 10.1093/cvr/cvad056. Cardiovasc Res. 2023. PMID: 37041718 Free PMC article.
-
Corticotropin-releasing hormone (CRH) depresses n-methyl-D-aspartate receptor-mediated current in cultured rat hippocampal neurons via CRH receptor type 1.Endocrinology. 2008 Mar;149(3):1389-98. doi: 10.1210/en.2007-1378. Epub 2007 Dec 13. Endocrinology. 2008. PMID: 18079206
-
Activation of CRF/CRFR1 Signaling in the Central Nucleus of the Amygdala Contributes to Chronic Stress-Induced Exacerbation of Neuropathic Pain by Enhancing GluN2B-NMDA Receptor-Mediated Synaptic Plasticity in Adult Male Rats.J Pain. 2024 Aug;25(8):104495. doi: 10.1016/j.jpain.2024.02.009. Epub 2024 Feb 12. J Pain. 2024. PMID: 38354968
-
Corticotrophin-releasing factor 1 activation in the central amygdale and visceral hyperalgesia.Neurogastroenterol Motil. 2015 Jan;27(1):1-6. doi: 10.1111/nmo.12495. Neurogastroenterol Motil. 2015. PMID: 25557223 Free PMC article. Review.
-
Glutamatergic transmission in opiate and alcohol dependence.Ann N Y Acad Sci. 2003 Nov;1003:196-211. doi: 10.1196/annals.1300.012. Ann N Y Acad Sci. 2003. PMID: 14684447 Review.
Cited by
-
A novel role of the antidepressant paroxetine in inhibiting neuronal Kv7/M channels to enhance neuronal excitability.Transl Psychiatry. 2025 Apr 2;15(1):116. doi: 10.1038/s41398-025-03291-w. Transl Psychiatry. 2025. PMID: 40175331 Free PMC article.
-
Pharmacological activation of the amygdala, but not single prolonged footshock-induced acute stress, interferes with cue-induced motivation toward food rewards in rats.Front Behav Neurosci. 2023 Sep 14;17:1252868. doi: 10.3389/fnbeh.2023.1252868. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37781505 Free PMC article.
-
Genetic Knockout of TRPM2 Increases Neuronal Excitability of Hippocampal Neurons by Inhibiting Kv7 Channel in Epilepsy.Mol Neurobiol. 2022 Nov;59(11):6918-6933. doi: 10.1007/s12035-022-02993-2. Epub 2022 Sep 2. Mol Neurobiol. 2022. PMID: 36053438
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

