Surgical Management for Chronic Destructive Septic Hip Arthritis: Debridement, Antibiotics, and Single-Stage Replacement is as Effective as Two-Stage Arthroplasty
- PMID: 35583090
- PMCID: PMC9163978
- DOI: 10.1111/os.13301
Surgical Management for Chronic Destructive Septic Hip Arthritis: Debridement, Antibiotics, and Single-Stage Replacement is as Effective as Two-Stage Arthroplasty
Abstract
Objective: To compare the surgical outcomes of debridement, antibiotics, and single-stage total hip replacement (DASR) vs two-stage arthroplasty (two-stage arthroplasty) for chronic destructive septic hip arthritis (SHA).
Methods: Cases of chronic destructive SHA treated by DASR or two-stage arthroplasty in our department from January 2008 to October 2021 were retrospectively reviewed. Patient demographic information, perioperative inflammation markers, intraoperative blood loss, microbial culture, and metagenomic new generation sequencing results were recorded. The perioperative complications, hospital stay, hospitalization cost, infection recurrence rate, and Harris Hip Score (HHS) at the last follow-up were compared between the two groups.
Results: A total of 28 patients were included in the study, including 11 patients who received DASR and 17 patients who received two-stage arthroplasty. There was no significant difference in demographic information, preoperative serum inflammatory markers, synovial fluid white blood cell count, or percentage of polymorphonuclear leukocytes between the two groups. The DASR group demonstrated significantly lower intraoperative blood loss [(368.2 ± 253.3) mL vs (638.2 ± 170.0) mL, p = 0.002], hospital stay [(22.6 ± 8.1) days vs (43.5 ± 13.2) days, p < 0.0001], and hospitalization expenses [(81,269 ± 11,496) RMB vs (137,524 ± 25,516) RMB, p < 0.0001] than the two-stage arthroplasty group. In the DASR group, one patient had dislocation as a complication. There were no cases with recurrence of infection. In the two-stage arthroplasty group, there was one case complicated with spacer fracture, one case with spacer dislocation, and one case with deep vein thrombosis of the lower limbs. There were no cases with recurrence of infection. There were no significant differences in the readmission rate, complication rate, or HHS at the last follow-up between the two groups.
Conclusions: Both DASR and two-stage arthroplasty achieved a satisfactory infection cure rate and functional recovery for chronic destructive SHA, and DASR demonstrated significantly lower intraoperative blood loss, hospital stay, and hospitalization costs than two-stage arthroplasty. For appropriately indicated patients, if microbial data are available and a standardized debridement protocol is strictly followed, DASR can be a treatment option.
Keywords: Debridement; Next generation sequencing; Septic arthritis; Total hip arthroplasty.
© 2022 The Authors. Orthopaedic Surgery published by Tianjin Hospital and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
None.
Figures


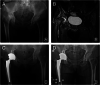

References
-
- Huang T‐W, Huang K‐C, Lee P‐C, Tai C‐L, Hsieh P‐H. Encouraging outcomes of staged, uncemented arthroplasty with short‐term antibiotic therapy for treatment of recalcitrant septic arthritis of the native hip. J Trauma. 2010;68:965–9. - PubMed
-
- Diwanji SR, Kong IK, Park YH, Cho SG, Song EK, Yoon TR. Two‐stage reconstruction of infected hip joints. J Arthroplasty. 2008;23:656–61. - PubMed
-
- Davis CM, Zamora RA. Surgical options and approaches for septic arthritis of the native hip and knee joint. J Arthroplasty. 2020;35:S14–8. - PubMed
-
- Stutz G, Kuster MS, Kleinstuck F, Gachter A. Arthroscopic management of septic arthritis: stages of infection and results. Knee Surg Sport Traumatol Arthrosc. 2000;8:270–4. - PubMed
MeSH terms
Substances
Grants and funding
- 2019-2020 Middle-aged and Young Experts with Outstanding Contributions to Health in Fujian Province
- 2019-ZQN-57/Fujian Provincial Health Technology Project
- 2019Y9122/Joint Funds for the innovation of science and Technology, Fujian province
- 2020I0015/Foreign Cooperation Project of Science and Technology, Fujian Province
- 82102621/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

