Demand and usage of unrelated donor products for allogeneic haematopoietic cell transplantation during the COVID-19 pandemic: A Canadian Blood Services Stem Cell Registry analysis
- PMID: 35583125
- PMCID: PMC9347538
- DOI: 10.1111/vox.13294
Demand and usage of unrelated donor products for allogeneic haematopoietic cell transplantation during the COVID-19 pandemic: A Canadian Blood Services Stem Cell Registry analysis
Abstract
Background and objectives: Understanding changes in the demand and usage of unrelated allogeneic haematopoietic cell transplantation (HCT) donors during the COVID-19 pandemic is needed to optimize pandemic preparedness of registry and donor collection services. The aim of this study was to understand the extent to which the pandemic has impacted the demand and usage of unrelated donors and cord blood units (CBUs) at Canadian Blood Services (CBS).
Materials and methods: Data regarding stem cell donor interest and product usage for unrelated allogeneic HCT were retrieved from the database at CBS using de-identified anonymous information.
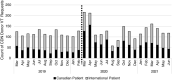
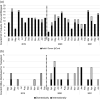
Results: Unrelated donor searches for Canadian patients remained unchanged by the pandemic, reflecting stable demand. The number of unrelated allogeneic transplants performed within Canada also remained stable, while the number of cord blood transplants increased, chiefly for paediatric patients. Requests for donor verification typing, a first signal of potential interest, increased from domestic centres during the first 6 months of the pandemic and decreased from international centres, before returning to baseline levels. The proportion of transplants for Canadian patients who used stem cell products procured from Canadian donors increased between 3 and 6 months after the start of the pandemic before returning to baseline and appears to be increasing again more than 1 year after the start of the pandemic. Use of CBUs for Canadian paediatric patients increased and remains elevated.
Conclusion: Demand for unrelated adult HCT donors has remained stable despite the evolving pandemic with a transient and recurring increased interest and usage of domestic adult donors. Use of CBUs for paediatric patients has increased and remains elevated. Registries and donor collection centres should maintain the capacity to expand services for domestic donor collection during pandemics to offset threats to international donor usage.
Keywords: COVID-19; cord blood; donor usage; haematopoietic cell transplantation; pandemic; unrelated donor.
© 2022 International Society of Blood Transfusion.
Conflict of interest statement
All authors are employed and/or paid consultants at Canadian Blood Services. There are no other conflicts of interest.
Figures

References
-
- Algwaiz G, Aljurf M, Koh M, Horowitz MM, Ljungman P, Weisdorf D, et al. Real‐world issues and potential solutions in hematopoietic cell transplantation during the COVID‐19 pandemic: perspectives from the worldwide network for blood and marrow transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol Blood Marrow Transplant. 2020;26:2181–9. - PMC - PubMed
-
- World Marrow Donor Association . COVID‐19 Impact on Registry Operations. Available from: https://share.wmda.info/display/LP/COVID-19+-+Impact+on+Registry+Operations. Accessed 28 Oct 2020.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

