Chirality-driven self-assembly: application toward renewable/exchangeable resin-immobilized catalysts
- PMID: 35583170
- PMCID: PMC9341390
- DOI: 10.1039/d2ob00439a
Chirality-driven self-assembly: application toward renewable/exchangeable resin-immobilized catalysts
Abstract
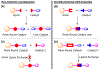
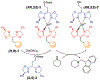
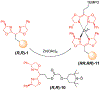
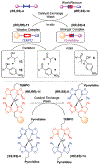
Resin-immobilized catalysts were prepared through chirality-driven self-assembly. The method allows the resin-immobilized catalyst to be regenerated under mild conditions and in situ catalyst exchange to be carried out quantitatively. The uniqueness of the methodology was demonstrated by the preparation of a catalyst for TEMPO oxidation as well as a two-step sequential TEMPO oxidation/aldol condensation sequence enabled by facile catalyst exchange.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

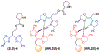
Similar articles
-
A trifunctional catalyst for one-pot synthesis of chiral diols via Heck coupling-N-oxidation-asymmetric dihydroxylation: application for the synthesis of diltiazem and taxol side chain.J Org Chem. 2003 Mar 7;68(5):1736-46. doi: 10.1021/jo026687i. J Org Chem. 2003. PMID: 12608786
-
Simple preparation and application of TEMPO-coated Fe(3)O(4) superparamagnetic nanoparticles for selective oxidation of alcohols.Chemistry. 2010 Nov 8;16(42):12718-26. doi: 10.1002/chem.200903527. Chemistry. 2010. PMID: 20853280
-
4-Amino-TEMPO-Immobilized Polymer Monolith: Preparations, and Recycling Performance of Catalyst for Alcohol Oxidation.Polymers (Basel). 2022 Nov 24;14(23):5123. doi: 10.3390/polym14235123. Polymers (Basel). 2022. PMID: 36501517 Free PMC article.
-
Recent advances in immobilized metal catalysts for environmentally benign oxidation of alcohols.Chem Asian J. 2008 Feb 1;3(2):196-214. doi: 10.1002/asia.200700359. Chem Asian J. 2008. PMID: 18232022 Review.
-
Molecular Catalyst Immobilized Photocathodes for Water/Proton and Carbon Dioxide Reduction.ChemSusChem. 2015 Nov;8(22):3746-59. doi: 10.1002/cssc.201500983. Epub 2015 Oct 6. ChemSusChem. 2015. PMID: 26437747 Review.
References
-
- Miceli M, Frontera P, Macario A and Malara A, Catalysts, 2021, 11, 591–607;
- Susam ZD and Tanyeli C, Asian J. Org. Chem, 2021, 10, 1251–1266;
- Liu M, Wu J and Hou H, Chem. – Eur. J, 2019, 25, 2935–2948; - PubMed
- Ye R, Zhukhovitskiy AV, Deraedt CV, Toste FD and Somorjai GA, Acc. Chem. Res, 2017, 50, 1894–1901; - PubMed
- Moberg C, Acc. Chem. Res, 2016, 49, 2736–2745. - PubMed
-
- Zhang H, Li H, Xu CC and Yang S, ACS Catal., 2019, 9, 10990–11029;
- Munnik P, de Jongh PE and de Jong KP, Chem. Rev, 2015, 115, 6687–6718; - PubMed
- Barak-Kulbak E, Goren K and Portnoy M, Pure Appl. Chem, 2014, 86, 1805–1818;
- Benaglia M, Puglisi A and Cozzi F, Chem. Rev, 2003, 103, 3401–3429. - PubMed
-
- Trindade AF, Gois PMP and Afonso CAM, Chem. Rev, 2009, 109, 418–514; - PubMed
- Lu J and Toy PH, Chem. Rev, 2009, 109, 815–838; - PubMed
- Heitbaum M, Glorius F and Escher I, Angew. Chem., Int. Ed, 2006, 45, 4732–4762; - PubMed
- Cozzi F, Adv. Synth. Catal, 2006, 348, 1367–1390;
- Benaglia M, New J. Chem, 2006, 30, 1525–1533.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

