Sirtuin modulators: past, present, and future perspectives
- PMID: 35583203
- PMCID: PMC9185222
- DOI: 10.4155/fmc-2022-0031
Sirtuin modulators: past, present, and future perspectives
Abstract
Sirtuins are NAD+-dependent protein lysine deacylase and mono-ADP ribosylases present in both prokaryotes and eukaryotes. The sirtuin family comprises seven isoforms in mammals, each possessing different subcellular localization and biological functions. Sirtuins have received increasing attention in the past two decades given their pivotal functions in a variety of biological contexts, including cytodifferentiation, transcriptional regulation, cell cycle progression, apoptosis, inflammation, metabolism, neurological and cardiovascular physiology and cancer. Consequently, modulation of sirtuin activity has been regarded as a promising therapeutic option for many pathologies. In this review, we provide an up-to-date overview of sirtuin biology and pharmacology. We examine the main features of the most relevant inhibitors and activators, analyzing their structure-activity relationships, applications in biology, and therapeutic potential.
Keywords: aging; cancer; drug discovery; epigenetics; metabolism; neurodegeneration; protein lysine deacylation; sirtuin modulators; sirtuins.
Conflict of interest statement
Financial & competing interests disclosure
This work was supported by FISR2019_00374 MeDyCa (A Mai), US National Institutes of Health n. R01GM114306 (A Mai), Progetto di Ateneo ‘Sapienza’ 2017 n. RM11715C7CA6CE53 and Regione Lazio Progetti di Gruppi di Ricerca 2020 – A0375-2020-36597 (D Rotili). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
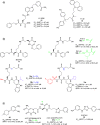
Figures





References
-
- Ho TCS, Chan AHY, Ganesan A. Thirty years of HDAC inhibitors: 2020 insight and hindsight. J. Med. Chem. 63(21), 12460–12484 (2020). - PubMed
-
- Mautone N, Zwergel C, Mai A, Rotili D. Sirtuin modulators: where are we now? A review of patents from 2015 to 2019. Expert Opin. Ther. Pat. 30(6), 389–407 (2020). - PubMed
-
- Fioravanti R, Mautone N, Rovere A et al. Targeting histone acetylation/deacetylation in parasites: an update (2017–2020). Curr. Opin. Chem. Biol. 57, 65–74 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
