Photoimmunotherapy Retains Its Anti-Tumor Efficacy with Increasing Stromal Content in Heterotypic Pancreatic Cancer Spheroids
- PMID: 35583476
- PMCID: PMC10443673
- DOI: 10.1021/acs.molpharmaceut.2c00260
Photoimmunotherapy Retains Its Anti-Tumor Efficacy with Increasing Stromal Content in Heterotypic Pancreatic Cancer Spheroids
Abstract
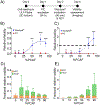
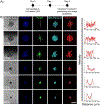
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease characterized by increased levels of desmoplasia that contribute to reduced drug delivery and poor treatment outcomes. In PDAC, the stromal content can account for up to 90% of the total tumor volume. The complex interplay between stromal components, including pancreatic cancer-associated fibroblasts (PCAFs), and PDAC cells in the tumor microenvironment has a significant impact on the prognoses and thus needs to be recapitulated in vitro when evaluating various treatment strategies. This study is a systematic evaluation of photodynamic therapy (PDT) in 3D heterotypic coculture models of PDAC with varying ratios of patient-derived PCAFs that simulate heterogeneous PDAC tumors with increasing stromal content. The efficacy of antibody-targeted PDT (photoimmunotherapy; PIT) using cetuximab (a clinically approved anti-EGFR antibody) photoimmunoconjugates (PICs) of a benzoporphyrin derivative (BPD) is contrasted with that of liposomal BPD (Visudyne), which is currently in clinical trials for PDT of PDAC. We demonstrate that both Visudyne-PDT and PIT were effective in heterotypic PDAC 3D spheroids with a low stromal content. However, as the stromal content increases above 50% in the 3D spheroids, the efficacy of Visudyne-PDT is reduced by up to 10-fold, while PIT retains its efficacy. PIT was found to be 10-, 19-, and 14-fold more phototoxic in spheroids with 50, 75, and 90% PCAFs, respectively, as compared to Visudyne-PDT. This marked difference in efficacy is attributed to the ability of PICs to penetrate and distribute homogeneously within spheroids with a higher stromal content and the mechanistically different modes of action of the two formulations. This study thus demonstrates how the stromal content in PDAC spheroids directly impacts their responsiveness to PDT and proposes PIT to be a highly suited treatment option for desmoplastic tumors with particularly high degrees of stromal content.
Keywords: Visudyne; benzoporphyrin derivative; cancer-associated fibroblasts; desmoplasia; epidermal growth factor receptor (EGFR); pancreatic ductal adenocarcinoma (PDAC); photodynamic therapy; photoimmunoconjugates; tumor microenvironment.
Conflict of interest statement
Conflict of Interest Statement:
The authors declare no competing financial interest.
Figures






References
-
- Siegel RL; Miller KD; Jemal A, Cancer statistics, 2020. CA 2020, 70 (1), 7–30. - PubMed
-
- Rahib L; Smith BD; Aizenberg R; Rosenzweig AB; Fleshman JM; Matrisian LM, Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res 2014, 74 (11), 2913–2921. - PubMed
-
- Grasso C; Jansen G; Giovannetti E, Drug resistance in pancreatic cancer: Impact of altered energy metabolism. Critical Reviews in Oncology/Hematology 2017, 114, 139–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

