Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge
- PMID: 35583664
- PMCID: PMC9348251
- DOI: 10.1111/ajt.17098
Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge
Abstract
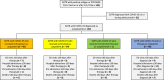
The SARS-CoV-2 pandemic continues to place a substantial burden on healthcare systems. Outpatient therapies for mild-to-moderate disease have reduced hospitalizations and deaths in clinical trials, but the real-world effectiveness of monoclonal antibodies and oral antiviral agents in solid organ transplant recipients (SOTR) with coronavirus disease-2019 (COVID-19) is largely uncharacterized. We conducted a single-center, retrospective review of 122 SOTR diagnosed with COVID-19 in the outpatient setting during the Omicron surge to address this knowledge gap. The mean age was 54 years, 57% were males, and 67% were kidney transplant recipients. The mean time from transplant to COVID-19 diagnosis was 75 months. Forty-nine (40%) received molnupiravir, 24 (20%) received sotrovimab, and 1 (0.8%) received nirmatrelvir/ritonavir. No outpatient therapy was administered in 48 (39%). All 122 SOTR had >30 days follow-up. Rates of hospitalization within 30 days of initiating therapy for molnupiravir, nirmatrelvir/ritonavir, and sotrovimab were 16% (8/49), 0% (0/1), and 8% (2/24), respectively, compared to 27% (13/48) in patients without outpatient therapy. There were no deaths in those who received any therapy versus 3 (6%) deaths in patients without outpatient therapy (p = .002). Overall, our experience suggests a role for monoclonal antibodies and oral antiviral agents in reducing COVID-19-related morbidity and mortality in SOTR.
Keywords: COVID-19; SARS-CoV-2; molnupiravir; nirmatrelvir; solid organ transplant; sotrovimab.
© 2022 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
References
-
- CDC. COVID Data Tracker. Published April 24, 2022. Accessed April 24, 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
-
- Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146–152. doi: 10.15585/mmwr.mm7104e4. doi: - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous