Biophysical physiology of phosphoinositide rapid dynamics and regulation in living cells
- PMID: 35583815
- PMCID: PMC9121023
- DOI: 10.1085/jgp.202113074
Biophysical physiology of phosphoinositide rapid dynamics and regulation in living cells
Abstract
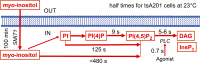
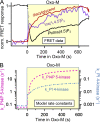
Phosphoinositide membrane lipids are ubiquitous low-abundance signaling molecules. They direct many physiological processes that involve ion channels, membrane identification, fusion of membrane vesicles, and vesicular endocytosis. Pools of these lipids are continually broken down and refilled in living cells, and the rates of some of these reactions are strongly accelerated by physiological stimuli. Recent biophysical experiments described here measure and model the kinetics and regulation of these lipid signals in intact cells. Rapid on-line monitoring of phosphoinositide metabolism is made possible by optical tools and electrophysiology. The experiments reviewed here reveal that as for other cellular second messengers, the dynamic turnover and lifetimes of membrane phosphoinositides are measured in seconds, controlling and timing rapid physiological responses, and the signaling is under strong metabolic regulation. The underlying mechanisms of this metabolic regulation remain questions for the future.
© 2022 Jensen et al.
Figures



Similar articles
-
Phosphoinositides and vesicular membrane traffic.Biochim Biophys Acta. 2012 Aug;1821(8):1104-13. doi: 10.1016/j.bbalip.2012.01.002. Epub 2012 Jan 14. Biochim Biophys Acta. 2012. PMID: 22281700 Free PMC article. Review.
-
Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways.Int J Mol Sci. 2017 Mar 15;18(3):634. doi: 10.3390/ijms18030634. Int J Mol Sci. 2017. PMID: 28294977 Free PMC article. Review.
-
Rho GTPases, phosphoinositides, and actin: a tripartite framework for efficient vesicular trafficking.Small GTPases. 2014;5:e29469. doi: 10.4161/sgtp.29469. Epub 2014 Jun 10. Small GTPases. 2014. PMID: 24914539 Free PMC article. Review.
-
Phosphoinositides: multipurpose cellular lipids with emerging roles in cell death.Cell Death Differ. 2019 May;26(5):781-793. doi: 10.1038/s41418-018-0269-2. Epub 2019 Feb 11. Cell Death Differ. 2019. PMID: 30742090 Free PMC article. Review.
-
Phosphoinositide switches in cell physiology - From molecular mechanisms to disease.J Biol Chem. 2024 Mar;300(3):105757. doi: 10.1016/j.jbc.2024.105757. Epub 2024 Feb 15. J Biol Chem. 2024. PMID: 38364889 Free PMC article. Review.
Cited by
-
A cell-permeable fluorescent probe reveals temporally diverse PI(4,5)P2 dynamics evoked by distinct GPCR agonists in neurons.Chem Sci. 2025 May 2;16(24):10970-10982. doi: 10.1039/d5sc01306b. eCollection 2025 Jun 18. Chem Sci. 2025. PMID: 40401190 Free PMC article.
-
PIP2 inhibits pore opening of the cyclic nucleotide-gated channel SthK.Nat Commun. 2024 Sep 19;15(1):8230. doi: 10.1038/s41467-024-52469-1. Nat Commun. 2024. PMID: 39300080 Free PMC article.
-
Allosteric stabilization of calcium and phosphoinositide dual binding engages several synaptotagmins in fast exocytosis.Elife. 2022 Aug 5;11:e74810. doi: 10.7554/eLife.74810. Elife. 2022. PMID: 35929728 Free PMC article.
-
Molecular architecture of the Gαi-bound TRPC5 ion channel.Nat Commun. 2023 May 3;14(1):2550. doi: 10.1038/s41467-023-38281-3. Nat Commun. 2023. PMID: 37137991 Free PMC article.
-
Electrocalcium coupling in brain capillaries: Rapidly traveling electrical signals ignite local calcium signals.Proc Natl Acad Sci U S A. 2024 Dec 17;121(51):e2415047121. doi: 10.1073/pnas.2415047121. Epub 2024 Dec 11. Proc Natl Acad Sci U S A. 2024. PMID: 39661063 Free PMC article.

