Hydration-mediated G-protein-coupled receptor activation
- PMID: 35584119
- PMCID: PMC9173805
- DOI: 10.1073/pnas.2117349119
Hydration-mediated G-protein-coupled receptor activation
Abstract
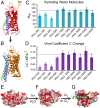
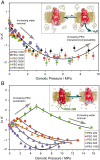
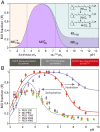
The Rhodopsin family of G-protein–coupled receptors (GPCRs) comprises the targets of nearly a third of all pharmaceuticals. Despite structural water present in GPCR X-ray structures, the physiological relevance of these solvent molecules to rhodopsin signaling remains unknown. Here, we show experimental results consistent with the idea that rhodopsin activation in lipid membranes is coupled to bulk water movements into the protein. To quantify hydration changes, we measured reversible shifting of the metarhodopsin equilibrium due to osmotic stress using an extensive series of polyethylene glycol (PEG) osmolytes. We discovered clear evidence that light activation entails a large influx of bulk water (∼80–100 molecules) into the protein, giving insight into GPCR activation mechanisms. Various size polymer osmolytes directly control rhodopsin activation, in which large solutes are excluded from rhodopsin and dehydrate the protein, favoring the inactive state. In contrast, small osmolytes initially forward shift the activation equilibrium until a quantifiable saturation point is reached, similar to gain-of-function protein mutations. For the limit of increasing osmolyte size, a universal response of rhodopsin to osmotic stress is observed, suggesting it adopts a dynamic, hydrated sponge-like state upon photoactivation. Our results demand a rethinking of the role of water dynamics in modulating various intermediates in the GPCR energy landscape. We propose that besides bound water, an influx of bulk water plays a necessary role in establishing the active GPCR conformation that mediates signaling.
Keywords: GPCR; osmotic stress; rhodopsin; sponge model; structural water.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Latorraca N. R., Venkatakrishnan A. J., Dror R. O., GPCR dynamics: Structures in motion. Chem. Rev. 117, 139–155 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

