Kir7.1 disease mutant T153I within the inner pore affects K+ conduction
- PMID: 35584325
- PMCID: PMC9273268
- DOI: 10.1152/ajpcell.00093.2022
Kir7.1 disease mutant T153I within the inner pore affects K+ conduction
Abstract
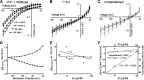
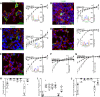
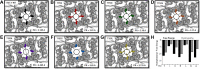
Inward-rectifier potassium channel 7.1 (Kir7.1) is present in the polarized epithelium, including the retinal pigmented epithelium. A single amino acid change at position 153 in the KCNJ13 gene, a substitution of threonine to isoleucine in the Kir7.1 protein, causes blindness. We hypothesized that the disease caused by this single amino acid substitution within the transmembrane protein domain could alter the translation, localization, or ion transport properties. We assessed the effects of amino acid side-chain length, arrangement, and polarity on channel structure and function. We showed that the T153I mutation yielded a full-length protein localized to the cell membrane. Whole cell patch-clamp recordings and chord conductance analyses revealed that the T153I mutant channel had negligible K+ conductance and failed to hyperpolarize the membrane potential. However, the mutant channel exhibited enhanced inward current when rubidium was used as a charge carrier, suggesting that an inner pore had formed and the channel was dysfunctional. Substituting with a polar, nonpolar, or short side-chain amino acid did not affect the localization of the protein. Still, it had an altered channel function due to differences in pore radius. Polar side chains (cysteine and serine) with inner pore radii comparable to wildtype exhibited normal inward K+ conductance. Short side chains (glycine and alanine) produced a channel with wider than expected inner pore size and lacked the biophysical characteristics of the wild-type channel. Leucine substitution produced results similar to the T153I mutant channel. This study provides direct electrophysiological evidence for the structure and function of the Kir7.1 channel's narrow inner pore in regulating conductance.
Keywords: Kir7.1; electrophysiology; innerpore structure; pediatric blindness; potassium channels.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Inward Rectifying K+ Channels.” Jerod Denton, PhD, and Eric Delpire, PhD, served as Guest Editors of this collection.
Figures



Similar articles
-
The unique structural characteristics of the Kir 7.1 inward rectifier potassium channel: a novel player in energy homeostasis control.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C694-C706. doi: 10.1152/ajpcell.00335.2022. Epub 2023 Jan 30. Am J Physiol Cell Physiol. 2023. PMID: 36717105 Free PMC article. Review.
-
Characterization of the R162W Kir7.1 mutation associated with snowflake vitreoretinopathy.Am J Physiol Cell Physiol. 2013 Mar 1;304(5):C440-9. doi: 10.1152/ajpcell.00363.2012. Epub 2012 Dec 19. Am J Physiol Cell Physiol. 2013. PMID: 23255580 Free PMC article.
-
Modulation of the Kir7.1 potassium channel by extracellular and intracellular pH.Am J Physiol Cell Physiol. 2008 Feb;294(2):C423-31. doi: 10.1152/ajpcell.00393.2007. Epub 2007 Dec 19. Am J Physiol Cell Physiol. 2008. PMID: 18094146
-
Expression and permeation properties of the K(+) channel Kir7.1 in the retinal pigment epithelium.J Physiol. 2001 Mar 1;531(Pt 2):329-46. doi: 10.1111/j.1469-7793.2001.0329i.x. J Physiol. 2001. PMID: 11230507 Free PMC article.
-
Focus on Kir7.1: physiology and channelopathy.Channels (Austin). 2014;8(6):488-95. doi: 10.4161/19336950.2014.959809. Channels (Austin). 2014. PMID: 25558901 Free PMC article. Review.
Cited by
-
The unique structural characteristics of the Kir 7.1 inward rectifier potassium channel: a novel player in energy homeostasis control.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C694-C706. doi: 10.1152/ajpcell.00335.2022. Epub 2023 Jan 30. Am J Physiol Cell Physiol. 2023. PMID: 36717105 Free PMC article. Review.
-
Special collection on inward rectifying K+ channels.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C603-C605. doi: 10.1152/ajpcell.00457.2022. Epub 2023 Jan 23. Am J Physiol Cell Physiol. 2023. PMID: 36689674 Free PMC article.
-
Inward rectifier potassium (Kir) channels in the retina: living our vision.Am J Physiol Cell Physiol. 2022 Sep 1;323(3):C772-C782. doi: 10.1152/ajpcell.00112.2022. Epub 2022 Aug 1. Am J Physiol Cell Physiol. 2022. PMID: 35912989 Free PMC article. Review.
-
Nonviral base editing of KCNJ13 mutation preserves vision in a model of inherited retinal channelopathy.J Clin Invest. 2023 Oct 2;133(19):e171356. doi: 10.1172/JCI171356. J Clin Invest. 2023. PMID: 37561581 Free PMC article.
References
-
- Nakamura N, Suzuki Y, Sakuta H, Ookata K, Kawahara K, Hirose S. Inwardly rectifying K+ channel Kir7.1 is highly expressed in thyroid follicular cells, intestinal epithelial cells and choroid plexus epithelial cells: implication for a functional coupling with Na+,K+-ATPase. Biochem J 342: 329–336, 1999. doi:10.1038/s41598-017-11034-1. - DOI - PMC - PubMed
-
- York N, Halbach P, Chiu MA, Bird IM, Pillers DM, Pattnaik BR. Oxytocin (OXT)-stimulated inhibition of Kir7.1 activity is through PIP2-dependent Ca(2+) response of the oxytocin receptor in the retinal pigment epithelium in vitro. Cell Signal 37: 93–102, 2017. doi:10.1016/j.cellsig.2017.06.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

