Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial
- PMID: 35584336
- PMCID: PMC9553388
- DOI: 10.1200/JCO.22.00338
Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial
Erratum in
-
Erratum: Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial.J Clin Oncol. 2023 Aug 10;41(23):3962. doi: 10.1200/JCO.23.01239. Epub 2023 Jun 26. J Clin Oncol. 2023. PMID: 37364222 No abstract available.
Abstract
Purpose: Patients with pretreated estrogen receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer have poor prognosis. Elacestrant is a novel, oral selective ER degrader that demonstrated activity in early studies.
Methods: This randomized, open-label, phase III trial enrolled patients with ER-positive/HER2-negative advanced breast cancer who had one-two lines of endocrine therapy, required pretreatment with a cyclin-dependent kinase 4/6 inhibitor, and ≤ 1 chemotherapy. Patients were randomly assigned to elacestrant 400 mg orally once daily or standard-of-care (SOC) endocrine monotherapy. Primary end points were progression-free survival (PFS) by blinded independent central review in all patients and patients with detectable ESR1 mutations.
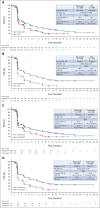
Results: Patients were randomly assigned to elacestrant (n = 239) or SOC (n = 238). ESR1 mutation was detected in 47.8% of patients, and 43.4% received two prior endocrine therapies. PFS was prolonged in all patients (hazard ratio = 0.70; 95% CI, 0.55 to 0.88; P = .002) and patients with ESR1 mutation (hazard ratio = 0.55; 95% CI, 0.39 to 0.77; P = .0005). Treatment-related grade 3/4 adverse events occurred in 7.2% receiving elacestrant and 3.1% receiving SOC. Treatment-related adverse events leading to treatment discontinuations were 3.4% in the elacestrant arm versus 0.9% in SOC. Nausea of any grade occurred in 35.0% receiving elacestrant and 18.8% receiving SOC (grade 3/4, 2.5% and 0.9%, respectively).
Conclusion: Elacestrant is the first oral selective ER degrader demonstrating a significant PFS improvement versus SOC both in the overall population and in patients with ESR1 mutations with manageable safety in a phase III trial for patients with ER-positive/HER2-negative advanced breast cancer.
Trial registration: ClinicalTrials.gov NCT03778931.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures


Comment in
-
Elacestrant and the Promise of Oral SERDs.J Clin Oncol. 2022 Oct 1;40(28):3227-3229. doi: 10.1200/JCO.22.00841. Epub 2022 Jun 23. J Clin Oncol. 2022. PMID: 35737918 No abstract available.
-
Oral Selective Estrogen Receptor Degrader After Endocrine Therapy With a Cyclin-Dependent Kinase 4/6 Inhibitor.J Clin Oncol. 2022 Dec 20;40(36):4280-4281. doi: 10.1200/JCO.22.01454. Epub 2022 Sep 6. J Clin Oncol. 2022. PMID: 36067450 No abstract available.
References
-
- National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 8.2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf - PubMed
-
- Gennari A, André F, Barrios CH, et al. : ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 32:1475-1495, 2021 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

