Burden of disease and associated complications of hepatitis a in children and adults in Mexico: A retrospective database study
- PMID: 35584365
- PMCID: PMC9116942
- DOI: 10.1371/journal.pone.0268469
Burden of disease and associated complications of hepatitis a in children and adults in Mexico: A retrospective database study
Abstract
Background: Hepatitis A virus (HAV) infection is a leading cause of viral hepatitis in children, yet the HAV vaccine is not included in the national immunization program (NIP) in Mexico. This study addresses an identified evidence gap of the burden of hepatitis A disease, complications, and associated costs in Mexico by analyzing surveillance and healthcare data. Data review included disease morbidity (incidence and hospitalization), mortality, and healthcare resource utilization costs.
Methods: In this observational, retrospective database study, we conducted a systematic screening, extraction, and analysis of outcome data from the national surveillance system in Mexico from January 2000 to December 2019.
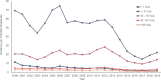
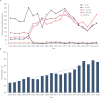
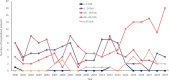
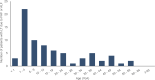
Results: During the analysis period (2000-2019), the average incidence rate/year of HAV cases was 14.7 (5.4-21.5) per 100,000 inhabitants. Children 1-9 years of age (YoA) had the highest average incidence rate/year with 47.8 (14.7-74.5). The average hospitalization rate/year due to HAV infection was 5.8% (2.9-9.6%). Although the highest burden of HAV continued to be in children (1-9 YoA), an increase in incidence and hospitalizations (with complications) in older age groups (≥ 10-64 YoA) was observed. The annual average fatality rate was estimated to be 0.44% (0.26-0.83%) of which 28.8% of deaths were concentrated in adults ≥ 65 YoA. The total direct costs of medical attention due to HAV and related complications were estimated at $382 million Mexican pesos.
Conclusion: The overall results suggest an uptrend in HAV infections in adolescents/adults compared to children in Mexico. Therefore, as the overall incidence risk of HAV infection decreases, the mean age of infection increases. This consequently increases the risk of severity and complications in older age groups, thus increasing the demand for healthcare resources. Our findings provide evidence for including the inactivated HAV vaccine in the Mexican NIP.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: AG-H, MYC-A, AA, and GH-G are employed by the GSK group of companies. MYC-A, AA, and GH-G hold shares from the GSK group of companies. GL-C and GS-G are owners of Estimatio SC and report that this company received support from the GSK group of companies to conduct this study. GS-G is an investigator at the Instituto Nacional de Salud Pública (INSP, Mexico). AG-H, MYC-A, AA, GH-G, GL-C, and GS-G declare no other financial and non-financial relationships and activities. ABG and VM-M declare no financial and non-financial relationships and activities and no conflicts of interest.
Figures


References
-
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases: Chapter 9 Hepatitis A. Washington D.C. Public Health Foundation; 2021. [cited 2021 Jun 22]Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html.
-
- Murphy TV, Feinstone SM, Bell BP. 14—Hepatitis A vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines (Sixth Edition). London: W.B. Saunders; 2013. p. 183–204.
-
- Perdigón-Villaseñor G F-CSB. Evolución reciente del comportamiento de las hepatitis virales en México, 1990–2007. Bol Med Hosp Infant Mex. 2009;66(2):204–207.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

