Ulinastatin alleviates early brain injury after intracerebral hemorrhage by inhibiting necroptosis and neuroinflammation via MAPK/NF-κB signaling pathway
- PMID: 35584533
- PMCID: PMC9109988
- DOI: 10.1590/acb370301
Ulinastatin alleviates early brain injury after intracerebral hemorrhage by inhibiting necroptosis and neuroinflammation via MAPK/NF-κB signaling pathway
Abstract
Purpose: Spontaneous intracerebral hemorrhage (ICH) is a major public health problem with a huge economic burden worldwide. Ulinastatin (UTI), a serine protease inhibitor, has been reported to be anti-inflammatory, immune regulation, and organ protection by reducing reactive oxygen species production, and inflammation. Necroptosis is a programmed cell death mechanism that plays a vital role in neuronal cell death after ICH. However, the neuroprotection of UTI in ICH has not been confirmed, and the potential mechanism is unclear. The present study aimed to investigate the neuroprotection and potential molecular mechanisms of UTI in ICH-induced EBI in a C57BL/6 mouse model.
Methods: The neurological score, brain water content, neuroinflammatory cytokine levels, and neuronal damage were evaluated. The anti-inflammation effectiveness of UTI in ICH patients also was evaluated.
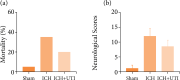
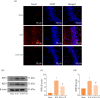
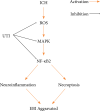
Results: UTI treatment markedly increased the neurological score, alleviate the brain edema, decreased the inflammatory cytokine TNF-α, interleukin‑1β (IL‑1β), IL‑6, NF‑κB levels, and RIP1/RIP3, which indicated that UTI-mediated inhibition of neuroinflammation, and necroptosis alleviated neuronal damage after ICH. UTI also can decrease the inflammatory cytokine of ICH patients. The neuroprotective capacity of UTI is partly dependent on the MAPK/NF-κB signaling pathway.
Conclusions: UTI improves neurological outcomes in mice and reduces neuronal death by protecting against neural neuroinflammation, and necroptosis.
Conflict of interest statement
Conflict of interest: Nothing to declare.
Figures

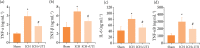


References
-
- Zhang Y, Zhang X, Wei Q, Leng S, Li C, Han B, Bai Y, Zhang H, Yao H. Activation of sigma-1 receptor enhanced pericyte survival via the interplay between apoptosis and autophagy: implications for blood-brain barrier integrity in stroke. Transl Stroke Res. 2020;11(2):267–287. doi: 10.1007/s12975-019-00711-0. - DOI - PubMed
-
- Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, Mayo SW, Bistran-Hall AJ, Gandhi D, Mould WA, Ullman N, Ali H, Carhuapoma JR, Kase CS, Lees KR, Dawson J, Wilson A, Betz JF, Sugar EA, Hao Y, Avadhani R, Caron JL, Harrigan MR, Carlson AP, Bulters D, LeDoux D, Huang J, Cobb C, Gupta G, Kitagawa R, Chicoine MR, Patel H, Dodd R, Camarata PJ, Wolfe S, Stadnik A, Money PL, Mitchell P, Sarabia R, Harnof S, Barzo P, Unterberg A, Teitelbaum JS, Wang W, Anderson CS, Mendelow AD, Gregson B, Janis S, Vespa P, Ziai W, Zuccarello M, Awad IA. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021–1032. doi: 10.1016/s0140-6736(19)30195-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

