The ciliary gene INPP5E confers dorsal telencephalic identity to human cortical organoids by negatively regulating Sonic hedgehog signaling
- PMID: 35584663
- PMCID: PMC9620745
- DOI: 10.1016/j.celrep.2022.110811
The ciliary gene INPP5E confers dorsal telencephalic identity to human cortical organoids by negatively regulating Sonic hedgehog signaling
Abstract
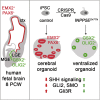
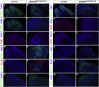
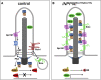
Defects in primary cilia, cellular antennas that control multiple intracellular signaling pathways, underlie several neurodevelopmental disorders, but it remains unknown how cilia control essential steps in human brain formation. Here, we show that cilia are present on the apical surface of radial glial cells in human fetal forebrain. Interfering with cilia signaling in human organoids by mutating the INPP5E gene leads to the formation of ventral telencephalic cell types instead of cortical progenitors and neurons. INPP5E mutant organoids also show increased Sonic hedgehog (SHH) signaling, and cyclopamine treatment partially rescues this ventralization. In addition, ciliary expression of SMO, GLI2, GPR161, and several intraflagellar transport (IFT) proteins is increased. Overall, these findings establish the importance of primary cilia for dorsal and ventral patterning in human corticogenesis, indicate a tissue-specific role of INPP5E as a negative regulator of SHH signaling, and have implications for the emerging roles of cilia in the pathogenesis of neurodevelopmental disorders.
Keywords: CP: Neuroscience; INPP5E; dorsal and ventral patterning; human cortex; primary cilia; sonic hedgehog; telencephalon.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

