Choice-selective sequences dominate in cortical relative to thalamic inputs to NAc to support reinforcement learning
- PMID: 35584665
- PMCID: PMC9218875
- DOI: 10.1016/j.celrep.2022.110756
Choice-selective sequences dominate in cortical relative to thalamic inputs to NAc to support reinforcement learning
Abstract
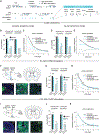
How are actions linked with subsequent outcomes to guide choices? The nucleus accumbens, which is implicated in this process, receives glutamatergic inputs from the prelimbic cortex and midline regions of the thalamus. However, little is known about whether and how representations differ across these input pathways. By comparing these inputs during a reinforcement learning task in mice, we discovered that prelimbic cortical inputs preferentially represent actions and choices, whereas midline thalamic inputs preferentially represent cues. Choice-selective activity in the prelimbic cortical inputs is organized in sequences that persist beyond the outcome. Through computational modeling, we demonstrate that these sequences can support the neural implementation of reinforcement-learning algorithms, in both a circuit model based on synaptic plasticity and one based on neural dynamics. Finally, we test and confirm a prediction of our circuit models by direct manipulation of nucleus accumbens input neurons.
Keywords: CP: Neuroscience; circuit modeling; imaging; learning; nucleus accumbens; optogenetics; prelimbic; reinforcement learning; thalamus.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





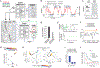
References
-
- Aggarwal M, Hyland BI, and Wickens JR (2012). Neural control of dopamine neurotransmission: implications for reinforcement learning. Eur. J. Neurosci 35, 1115–1123. - PubMed
-
- Apicella P, Ljungberg T, Scarnati E, and Schultz W (1991). Responses to reward in monkey dorsal and ventral striatum. Exp. Brain Res 85, 491–500. - PubMed
-
- Atallah HE, Lopez-Paniagua D, Rudy JW, and O’Reilly RC (2007). Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat. Neurosci 10, 126–131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

