The non-essential TSC complex component TBC1D7 restricts tissue mTORC1 signaling and brain and neuron growth
- PMID: 35584673
- PMCID: PMC9175135
- DOI: 10.1016/j.celrep.2022.110824
The non-essential TSC complex component TBC1D7 restricts tissue mTORC1 signaling and brain and neuron growth
Abstract
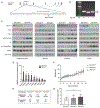
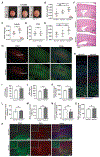
The tuberous sclerosis complex (TSC) 1 and 2 proteins associate with TBC1D7 to form the TSC complex, which is an essential suppressor of mTOR complex 1 (mTORC1), a ubiquitous driver of cell and tissue growth. Loss-of-function mutations in TSC1 or TSC2, but not TBC1D7, give rise to TSC, a pleiotropic disorder with aberrant activation of mTORC1 in various tissues. Here, we characterize mice with genetic deletion of Tbc1d7, which are viable with normal growth and development. Consistent with partial loss of function of the TSC complex, Tbc1d7 knockout (KO) mice display variable increases in tissue mTORC1 signaling with increased muscle fiber size but with strength and motor defects. Their most pronounced phenotype is brain overgrowth due to thickening of the cerebral cortex, with enhanced neuron-intrinsic mTORC1 signaling and growth. Thus, TBC1D7 is required for full TSC complex function in tissues, and the brain is particularly sensitive to its growth-suppressing activities.
Keywords: CP: Developmental biology; CP: Neuroscience; TBC1D7; Tsc1; Tsc2; brain; gait; growth; hamartin; mTOR; megalencephaly; mouse model; neurons; rapamycin; tuberin; tuberous sclerosis complex.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.D.M. is a member of the scientific advisory board and a shareholder of Navitor Pharmaceuticals. M.S. reports grant support from Novartis, Biogen, Astellas, Aeovian, Bridgebio, and Aucta and is on the scientific advisory boards for Novartis, Roche, Regenxbio, SpringWorks Therapeutics, Jaguar Therapeutics, and Alkermes. All other authors declare no competing financial interests.
Figures


References
-
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, and Sabatini DM (2013). A tumor suppressor complex with GAP activity for the rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100–1106. 10.1126/science.1232044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

