Granule Cells Constitute One of the Major Neuronal Subtypes in the Molecular Layer of the Posterior Cerebellum
- PMID: 35584915
- PMCID: PMC9172288
- DOI: 10.1523/ENEURO.0289-21.2022
Granule Cells Constitute One of the Major Neuronal Subtypes in the Molecular Layer of the Posterior Cerebellum
Abstract
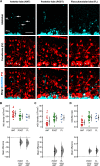
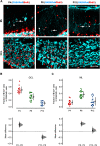
The migration of neurons from their birthplace to their correct destination is one of the most crucial steps in brain development. Incomplete or incorrect migration yields ectopic neurons, which cause neurologic deficits or are negligible at best. However, the granule cells (GCs) in the cerebellar cortex may challenge this traditional view of ectopic neurons. When animals are born, GCs proliferate near the pia mater and then migrate down to the GC layer located deep in the cerebellar cortex. However, some GC-like cells stay in the molecular layer, a layer between the pia mater and GC layer, even in normal adult animals. These cells were named ectopic GCs nearly 50 years ago, but their abundance and functional properties remain unclear. Here, we have examined GCs in the molecular layer (mGCs) with a specific marker for mature GCs and transgenic mice in which GCs are sparsely labeled with a fluorescent protein. Contrary to the previous assumption that mGCs are a minor neuronal population, we have found that mGCs are as prevalent as stellate or basket cells in the posterior cerebellum. They are produced during a similar period as regular GCs (rGCs), and in vivo time-lapse imaging has revealed that mGCs are stably present in the molecular layer. Whole-cell patch-clamp recordings have shown that mGCs discharge action potentials similarly to rGCs. Since axonal inputs differ between the molecular layer and GC layer, mGCs might be incorporated in different micro-circuits from rGCs and have a unique functional role in the cerebellum.
Keywords: cerebellum; development; ectopic neurons; granule cells; migration.
Copyright © 2022 Dey et al.
Figures




References
-
- Altman J, Bayer SA (1996) Development of the cerebellar system: in relation to its evolution, structure, and functions, Ed 1. Boca Raton: CRC-Press.
-
- Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, Ebner TJ, Isope P, Jörntell H, Lackey EP, Lawrenson C, Lumb B, Schonewille M, Sillitoe RV, Spaeth L, Sugihara I, Valera A, Voogd J, Wylie DR, Ruigrok TJH (2018) Cerebellar modules and their role as operational cerebellar processing units. Cerebellum 17:654–682. 10.1007/s12311-018-0952-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
