Programmed cell death in atherosclerosis and vascular calcification
- PMID: 35585052
- PMCID: PMC9117271
- DOI: 10.1038/s41419-022-04923-5
Programmed cell death in atherosclerosis and vascular calcification
Abstract
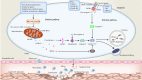
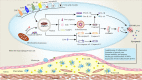
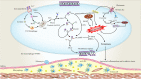
The concept of cell death has been expanded beyond apoptosis and necrosis to additional forms, including necroptosis, pyroptosis, autophagy, and ferroptosis. These cell death modalities play a critical role in all aspects of life, which are noteworthy for their diverse roles in diseases. Atherosclerosis (AS) and vascular calcification (VC) are major causes for the high morbidity and mortality of cardiovascular disease. Despite considerable advances in understanding the signaling pathways associated with AS and VC, the exact molecular basis remains obscure. In the article, we review the molecular mechanisms that mediate cell death and its implications for AS and VC. A better understanding of the mechanisms underlying cell death in AS and VC may drive the development of promising therapeutic strategies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Li M, Zhu Y, Jaiswal SK, Liu NF. Mitochondria homeostasis and vascular medial calcification. Calcif Tissue Int. 2021;109:113–20. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

