Structural basis for inhibition of the Cation-chloride cotransporter NKCC1 by the diuretic drug bumetanide
- PMID: 35585053
- PMCID: PMC9117670
- DOI: 10.1038/s41467-022-30407-3
Structural basis for inhibition of the Cation-chloride cotransporter NKCC1 by the diuretic drug bumetanide
Abstract
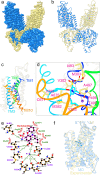
Cation-chloride cotransporters (CCCs) NKCC1 and NKCC2 catalyze electroneutral symport of 1 Na+, 1 K+, and 2 Cl- across cell membranes. NKCC1 mediates trans-epithelial Cl- secretion and regulates excitability of some neurons and NKCC2 is critical to renal salt reabsorption. Both transporters are inhibited by the so-called loop diuretics including bumetanide, and these drugs are a mainstay for treating edema and hypertension. Here, our single-particle electron cryo-microscopy structures supported by functional studies reveal an outward-facing conformation of NKCC1, showing bumetanide wedged into a pocket in the extracellular ion translocation pathway. Based on these and the previously published inward-facing structures, we define the translocation pathway and the conformational changes necessary for ion translocation. We also identify an NKCC1 dimer with separated transmembrane domains and extensive transmembrane and C-terminal domain interactions. We further define an N-terminal phosphoregulatory domain that interacts with the C-terminal domain, suggesting a mechanism whereby (de)phosphorylation regulates NKCC1 by tuning the strength of this domain association.
© 2022. The Author(s).
Conflict of interest statement
Laura Cancedda and Marco De Vivo are founders of the IAMA Therapeutics. All other authors declare no competing interests.
Figures



Similar articles
-
Structural basis for human NKCC1 inhibition by loop diuretic drugs.EMBO J. 2025 Mar;44(5):1540-1562. doi: 10.1038/s44318-025-00368-6. Epub 2025 Jan 28. EMBO J. 2025. PMID: 39875725 Free PMC article.
-
Loop diuretic and ion-binding residues revealed by scanning mutagenesis of transmembrane helix 3 (TM3) of Na-K-Cl cotransporter (NKCC1).J Biol Chem. 2012 May 18;287(21):17308-17317. doi: 10.1074/jbc.M112.356014. Epub 2012 Mar 21. J Biol Chem. 2012. PMID: 22437837 Free PMC article.
-
The search for NKCC1-selective drugs for the treatment of epilepsy: Structure-function relationship of bumetanide and various bumetanide derivatives in inhibiting the human cation-chloride cotransporter NKCC1A.Epilepsy Behav. 2016 Jun;59:42-9. doi: 10.1016/j.yebeh.2016.03.021. Epub 2016 Apr 15. Epilepsy Behav. 2016. PMID: 27088517
-
Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters.Physiol Rev. 2005 Apr;85(2):423-93. doi: 10.1152/physrev.00011.2004. Physiol Rev. 2005. PMID: 15788703 Review.
-
CNS pharmacology of NKCC1 inhibitors.Neuropharmacology. 2022 Mar 1;205:108910. doi: 10.1016/j.neuropharm.2021.108910. Epub 2021 Dec 6. Neuropharmacology. 2022. PMID: 34883135 Review.
Cited by
-
Cryo-EM structure of the human sodium-chloride cotransporter NCC.Sci Adv. 2022 Nov 11;8(45):eadd7176. doi: 10.1126/sciadv.add7176. Epub 2022 Nov 9. Sci Adv. 2022. PMID: 36351028 Free PMC article.
-
Molecular mechanisms of thiazide-like diuretics-mediated inhibition of the human Na-Cl cotransporter.Nat Commun. 2025 Aug 20;16(1):7740. doi: 10.1038/s41467-025-62714-w. Nat Commun. 2025. PMID: 40830368 Free PMC article.
-
Intestinal organ chips for disease modelling and personalized medicine.Nat Rev Gastroenterol Hepatol. 2024 Nov;21(11):751-773. doi: 10.1038/s41575-024-00968-3. Epub 2024 Aug 27. Nat Rev Gastroenterol Hepatol. 2024. PMID: 39192055 Review.
-
Chloride Ions, Vascular Function and Hypertension.Biomedicines. 2022 Sep 18;10(9):2316. doi: 10.3390/biomedicines10092316. Biomedicines. 2022. PMID: 36140417 Free PMC article. Review.
-
Kidney and blood pressure regulation-latest evidence for molecular mechanisms.Clin Kidney J. 2023 Jan 24;16(6):952-964. doi: 10.1093/ckj/sfad015. eCollection 2023 Jun. Clin Kidney J. 2023. PMID: 37261007 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

