Novel role of the synaptic scaffold protein Dlgap4 in ventricular surface integrity and neuronal migration during cortical development
- PMID: 35585091
- PMCID: PMC9117333
- DOI: 10.1038/s41467-022-30443-z
Novel role of the synaptic scaffold protein Dlgap4 in ventricular surface integrity and neuronal migration during cortical development
Abstract
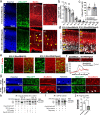
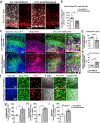
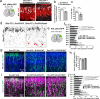
Subcortical heterotopias are malformations associated with epilepsy and intellectual disability, characterized by the presence of ectopic neurons in the white matter. Mouse and human heterotopia mutations were identified in the microtubule-binding protein Echinoderm microtubule-associated protein-like 1, EML1. Further exploring pathological mechanisms, we identified a patient with an EML1-like phenotype and a novel genetic variation in DLGAP4. The protein belongs to a membrane-associated guanylate kinase family known to function in glutamate synapses. We showed that DLGAP4 is strongly expressed in the mouse ventricular zone (VZ) from early corticogenesis, and interacts with key VZ proteins including EML1. In utero electroporation of Dlgap4 knockdown (KD) and overexpression constructs revealed a ventricular surface phenotype including changes in progenitor cell dynamics, morphology, proliferation and neuronal migration defects. The Dlgap4 KD phenotype was rescued by wild-type but not mutant DLGAP4. Dlgap4 is required for the organization of radial glial cell adherens junction components and actin cytoskeleton dynamics at the apical domain, as well as during neuronal migration. Finally, Dlgap4 heterozygous knockout (KO) mice also show developmental defects in the dorsal telencephalon. We hence identify a synapse-related scaffold protein with pleiotropic functions, influencing the integrity of the developing cerebral cortex.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
The neuroanatomy of Eml1 knockout mice, a model of subcortical heterotopia.J Anat. 2019 Sep;235(3):637-650. doi: 10.1111/joa.13013. Epub 2019 Jun 7. J Anat. 2019. PMID: 31173351 Free PMC article.
-
Eml1 loss impairs apical progenitor spindle length and soma shape in the developing cerebral cortex.Sci Rep. 2017 Dec 11;7(1):17308. doi: 10.1038/s41598-017-15253-4. Sci Rep. 2017. PMID: 29229923 Free PMC article.
-
Impairment of radial glial scaffold-dependent neuronal migration and formation of double cortex by genetic ablation of afadin.Brain Res. 2015 Sep 16;1620:139-52. doi: 10.1016/j.brainres.2015.05.012. Epub 2015 May 16. Brain Res. 2015. PMID: 25988834
-
The DLGAP family: neuronal expression, function and role in brain disorders.Mol Brain. 2017 Sep 4;10(1):43. doi: 10.1186/s13041-017-0324-9. Mol Brain. 2017. PMID: 28870203 Free PMC article. Review.
-
Genes that regulate neuronal migration in the cerebral cortex.Epilepsy Res. 1999 Sep;36(2-3):143-54. doi: 10.1016/s0920-1211(99)00048-0. Epilepsy Res. 1999. PMID: 10515162 Review.
Cited by
-
Radial glia progenitor polarity in health and disease.Front Cell Dev Biol. 2024 Oct 2;12:1478283. doi: 10.3389/fcell.2024.1478283. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39416687 Free PMC article. Review.
-
Inhibition of Foxp4 Disrupts Cadherin-based Adhesion of Radial Glial Cells, Leading to Abnormal Differentiation and Migration of Cortical Neurons in Mice.Neurosci Bull. 2023 Jul;39(7):1131-1145. doi: 10.1007/s12264-022-01004-7. Epub 2023 Jan 16. Neurosci Bull. 2023. PMID: 36646976 Free PMC article.
-
SAPAP Scaffold Proteins: From Synaptic Function to Neuropsychiatric Disorders.Cells. 2022 Nov 28;11(23):3815. doi: 10.3390/cells11233815. Cells. 2022. PMID: 36497075 Free PMC article. Review.
-
Non-synaptic function of the autism spectrum disorder-associated gene SYNGAP1 in cortical neurogenesis.Nat Neurosci. 2023 Dec;26(12):2090-2103. doi: 10.1038/s41593-023-01477-3. Epub 2023 Nov 9. Nat Neurosci. 2023. PMID: 37946050 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

