Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions
- PMID: 35585199
- PMCID: PMC9116713
- DOI: 10.1038/s41574-022-00683-6
Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions
Abstract
Glucocorticoid hormones were discovered to have use as potent anti-inflammatory and immunosuppressive therapeutics in the 1940s and their continued use and development have successfully revolutionized the management of acute and chronic inflammatory diseases. However, long-term use of glucocorticoids is severely hampered by undesirable metabolic complications, including the development of type 2 diabetes mellitus. These effects occur due to glucocorticoid receptor activation within multiple tissues, which results in inter-organ crosstalk that increases hepatic glucose production and inhibits peripheral glucose uptake. Despite the high prevalence of glucocorticoid-induced hyperglycaemia associated with their routine clinical use, treatment protocols for optimal management of the metabolic adverse effects are lacking or underutilized. The type, dose and potency of the glucocorticoid administered dictates the choice of hypoglycaemic intervention (non-insulin or insulin therapy) that should be provided to patients. The longstanding quest to identify dissociated glucocorticoid receptor agonists to separate the hyperglycaemic complications of glucocorticoids from their therapeutically beneficial anti-inflammatory effects is ongoing, with selective glucocorticoid receptor modulators in clinical testing. Promising areas of preclinical research include new mechanisms to disrupt glucocorticoid signalling in a tissue-selective manner and the identification of novel targets that can selectively dissociate the effects of glucocorticoids. These research arms share the ultimate goal of achieving the anti-inflammatory actions of glucocorticoids without the metabolic consequences.
© 2022. Crown.
Conflict of interest statement
C.L.C. is a co-inventor on US9428753 patent entitled “Use of LXR antagonists for treatment of side effects of elevated glucocorticoid levels”. J.X.L. declares no competing interests.
Figures

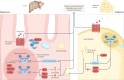
References
-
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. - PubMed
-
- Hench PS, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc. Staff. Meet. Mayo Clin. 1949;24:181–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

