Effect of PEG grafting density on surface properties of polyurethane substrata and the viability of osteoblast and fibroblast cells
- PMID: 35585216
- PMCID: PMC9117377
- DOI: 10.1007/s10856-022-06668-1
Effect of PEG grafting density on surface properties of polyurethane substrata and the viability of osteoblast and fibroblast cells
Abstract
The surface of Tecoflex SG-80A Polyurethane (PU) films was modified by grafting polyethylene glycol (PEG) chains at three different molar amounts (0.05, 0.10, and 0.15 mmol). The resulting substrata were characterized by FTIR-ATR, TGA, AFM, SEM and contact angle to assess the surface modifications occurred during the grafting reactions. Osteoblasts and fibroblasts were cultured with PU extracts for 24 h, and their cell viability and morphology were evaluated by CellTiterBlue assay, Crystal Violet staining and Live/Dead assay. FTIR and TGA results indicated that PEG chains were successfully grafted onto PU surfaces, specifically in the hard segment of PU forming allophanate groups as the PEG grafting density increased. SEM and AFM images suggest that PU substrata were partially covered by PEG, increasing the dispersive and basic components of the PU surface energy. It was found that extracts from PEG-grafted polyurethanes increased the osteoblast viability, although fibroblasts viability remained constant regardless PEG grafting density; in spite of this both cells presented a more spread morphology at the lower PEG grafting density. Our results showed that surface energy of PU substrata can be tuned by PEG grafting density; also, the PEG leached tends to increase the pH of culture medium which leads to a higher viability of osteoblasts; nevertheless, PEG grafting density should be optimized to promote a healthy cell morphology as alterations in its morphology were detected at higher concentrations. Graphical abstract.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
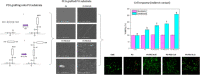

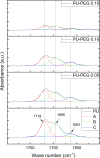



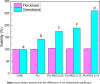



Similar articles
-
Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts.Polymers (Basel). 2022 Nov 14;14(22):4912. doi: 10.3390/polym14224912. Polymers (Basel). 2022. PMID: 36433040 Free PMC article.
-
Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale.Biomaterials. 2003 Nov;24(25):4655-61. doi: 10.1016/s0142-9612(03)00361-2. Biomaterials. 2003. PMID: 12951008
-
Platelet adhesion and activation on polyethylene glycol modified polyurethane surfaces. Measurement of cytoplasmic calcium.ASAIO J. 1996 Sep-Oct;42(5):M876-81. doi: 10.1097/00002480-199609000-00117. ASAIO J. 1996. PMID: 8945010
-
Synthesis, Characterization, and Bacterial Fouling-Resistance Properties of Polyethylene Glycol-Grafted Polyurethane Elastomers.Int J Mol Sci. 2019 Feb 25;20(4):1001. doi: 10.3390/ijms20041001. Int J Mol Sci. 2019. PMID: 30823606 Free PMC article.
-
Preparation and surface properties of PEO-sulfonate grafted polyurethanes for enhanced blood compatibility.J Biomater Sci Polym Ed. 1993;4(6):579-89. doi: 10.1163/156856293x00221. J Biomater Sci Polym Ed. 1993. PMID: 8280672
Cited by
-
Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts.Polymers (Basel). 2022 Nov 14;14(22):4912. doi: 10.3390/polym14224912. Polymers (Basel). 2022. PMID: 36433040 Free PMC article.
-
Effect of Sterilization Methods on Electrospun Scaffolds Produced from Blend of Polyurethane with Gelatin.J Funct Biomater. 2023 Jan 28;14(2):70. doi: 10.3390/jfb14020070. J Funct Biomater. 2023. PMID: 36826869 Free PMC article.
-
A paradigm for high-throughput screening of cell-selective surfaces coupling orthogonal gradients and machine learning-based cell recognition.Bioact Mater. 2023 May 5;28:1-11. doi: 10.1016/j.bioactmat.2023.04.022. eCollection 2023 Oct. Bioact Mater. 2023. PMID: 37214260 Free PMC article.
-
Recent Advances in Polyurethane for Artificial Vascular Application.Polymers (Basel). 2024 Dec 18;16(24):3528. doi: 10.3390/polym16243528. Polymers (Basel). 2024. PMID: 39771380 Free PMC article. Review.
References
-
- Agarwal R, García AJ. Surface modification of biomaterials. Princ Regen Med. 2019:651–60. 10.1016/b978-0-12-809880-6.00037-0.
-
- Chen S, Guo Y, Liu R, Wu S, Fang J, Huang B, et al. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf B Biointerfaces. 2018;164:58–69. doi: 10.1016/j.colsurfb.2018.01.022. - DOI - PubMed
-
- Hoshiba T, Yoshihiro A, Tanaka M. Evaluation of initial cell adhesion on poly (2- methoxyethyl acrylate) (PMEA) analogous polymers. J Biomater Sci Polym Ed. 2017;5063. 10.1080/09205063.2017.1312738. - PubMed
-
- Tidwell CD, Ertel SI, Ratner BD, Tarasevich BJ, Atre S, Allara DL. Endothelial cell growth and protein adsorption on terminally functionalized, self-assembled monolayers of alkanethiolates on gold. Langmuir. 1997;13:3404–13. doi: 10.1021/la9604341. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

