Results of screening in early and advanced thoracic malignancies in the EORTC pan-European SPECTAlung platform
- PMID: 35585228
- PMCID: PMC9117328
- DOI: 10.1038/s41598-022-12056-0
Results of screening in early and advanced thoracic malignancies in the EORTC pan-European SPECTAlung platform
Abstract
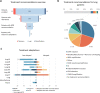
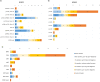
Access to a comprehensive molecular alteration screening is patchy in Europe and quality of the molecular analysis varies. SPECTAlung was created in 2015 as a pan-European screening platform for patients with thoracic malignancies. Here we report the results of almost 4 years of prospective molecular screening of patients with thoracic malignancies, in terms of quality of the program and molecular alterations identified. Patients with thoracic malignancies at any stage of disease were recruited in SPECTAlung, from June 2015 to May 2019, in 7 different countries. Molecular tumour boards were organised monthly to discuss patients' molecular and clinical profile and possible biomarker-driven treatments, including clinical trial options. FFPE material was collected and analysed for 576 patients with diagnosis of pleural, lung, or thymic malignancies. Ultimately, 539 patients were eligible (93.6%) and 528 patients were assessable (91.7%). The turn-around time for report generation and molecular tumour board was 214 days (median). Targetable molecular alterations were observed in almost 20% of cases, but treatment adaptation was low (3% of patients). SPECTAlung showed the feasibility of a pan-European screening platform. One fifth of the patients had a targetable molecular alteration. Some operational issues were discovered and adapted to improve efficiency.
© 2022. The Author(s).
Conflict of interest statement
Dr. Morfouace, A. Stevovic, Dr. Gorlia, Dr. Golfinopoulos, Dr. Dooms, Dr. Janžič, Dr. Berghmans, Dr. O’Brien, Dr. Bironzo, Dr. Vansteenkiste and Dr. Lacroix declare no competing interests. Dr. MAZIERES reports: Personal fees from Roche, Astra Zeneca, Pierre Fabre, Takeda, BMS, MSD, Jiangsu Hengrui, Blueprint, Daiichi, Novartis, Amgen. Grants from Roche, Astra Zeneca, Pierre Fabre, BMS. Dr. DINGEMANS reports: Attending advisory boards and/or provided lectures for: Roche, Eli Lilly, Boehringer Ingelheim, Astra Zeneca, Pfizer, BMS, Amgen, Novartis, MSD, Takeda, Pharmamar. Receiving research support from BMS, AbbVie, Amgen (all paid to her institute). Dr. NOVELLO reports: Being an advisor/Speaker bureau: AZ, AMG, BI, Beigene, MSD, Eli Lilly, Roche, Takeda, Pfizer, Roche, Sanofi, Novartis. Dr. FELIP reports: Advisory Role or Speaker’s Bureau: Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Glaxo Smith Kline, Janssen, Medscape, Merck KGaA, Merck Sharp & Dohme, Novartis, Peptomyc, PeerVoice, Pfizer, Regeneron, Sanofi Genzyme, Syneos Health, Seattle Genetics, Takeda, Touch Medical. Board member: Grifols, Independent member. Research Funding: Fundación Merck Salud, Grant for Oncology Innovation, Merck Healthcare KGaA. Dr. PAZ-ARES reports: Grants or contracts from any entity: MSD, Astrazeneca, Pfizer, BMS. Consulting fees: Lilly, MSD, Roche, Pharmamar, Merck, Astrazeneca, Novartis, Servier, Amgen, Pfizer, Ipsen, Sanofi, Bayer, Blueprint, BMS, Mirati. Payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events: Astrazeneca, Janssen, Merck, Mirati, Sanofi. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Genomica, Altum sequency. Dr. DZIADZUSZKO reports: Advisory role/compensated: AstraZeneca, Roche, Novartis, MSD, Pfizer, Boehringer Ingelheim, FoundationMedicine, Karyopharm, Takeda. Dr. BESSE reports: Sponsored Research at Gustave Roussy Cancer Center: 4D Pharma, Abbvie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Inivata, Janssen, Onxeo, OSE immunotherapeutics, Pfizer, Roche-Genentech, Sanofi, Takeda, Tolero Pharmaceuticals.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

