Curative resection after chemotherapy and chemoradiotherapy for postoperative recurrence of pancreatic tail cancer in the abdominal wall: a case report
- PMID: 35585274
- PMCID: PMC9117584
- DOI: 10.1186/s40792-022-01452-3
Curative resection after chemotherapy and chemoradiotherapy for postoperative recurrence of pancreatic tail cancer in the abdominal wall: a case report
Abstract
Background: Locoregional recurrence and metastasis to the liver, peritoneum, and lung are the most common recurrent patterns of pancreatic ductal adenocarcinoma (PDAC) after radical resection. Recurrence in the abdominal wall is extremely rare. Herein, we report our experience with a patient who had recurrent PDAC in the abdominal wall with long-term survival by means of multidisciplinary therapy.
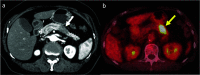
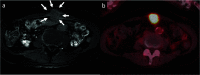
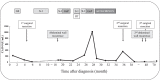
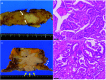
Case presentation: A 76-year-old Japanese woman was diagnosed with resectable pancreatic tail cancer. She underwent distal pancreatectomy with regional lymphadenectomy after two cycles of gemcitabine plus S-1 as neoadjuvant therapy. She also received eight cycles of S-1 as adjuvant chemotherapy. Approximately 14 months after the initial surgery, imaging examinations identified a mass suggesting recurrence in the abdominal wall at the middle wound that involved the transverse colon. After two cycles of gemcitabine plus nab-paclitaxel, chemoradiotherapy (S-1 plus 45 Gy) and seven cycles of modified FOLFIRINOX (5-fluorouracil/leucovorin, irinotecan, and oxaliplatin) were administered. The patient did not develop any new recurrent lesions during chemotherapy and chemoradiotherapy. Therefore, the recurrent lesion in the abdominal wall and the involved transverse colon were resected. We confirmed the lack of peritoneal dissemination during surgery. Pathological examination revealed that the resected lesion was metastasis of primary PDAC, and the surgical margin was 1 mm. However, re-recurrence localized in the abdominal wall was detected 9 months later. The re-recurrent lesion was diagnosed as local recurrence of the first recurrent lesion. We performed a second resection of the abdominal wall using a femoral myocutaneous flap to achieve sufficient surgical margin. The pathological findings of the resected specimen were the same as those of the previous specimens, and the resection margin was negative. The patient's postoperative course was uneventful. Seven years after the initial surgery and 3 years and 7 months after the third surgery, the patient is alive with no signs of recurrence.
Conclusions: Long-term survival could be achieved by radical resection with sufficient surgical margins for recurrence of PDAC in the abdominal wall if new other recurrent lesions, including peritoneal dissemination, are prevented through chemotherapy.
Keywords: Abdominal wall recurrence; Femoral myocutaneous flap; Multidisciplinary therapy; Pancreatic ductal adenocarcinoma; Surgical margin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Synchronous double primary malignancies of the pancreatic body and extrahepatic bile duct treated with pancreatoduodenectomy and splenic artery resection following neoadjuvant chemotherapy with gemcitabine plus nab-paclitaxel: a case report.Surg Case Rep. 2022 Feb 16;8(1):29. doi: 10.1186/s40792-022-01383-z. Surg Case Rep. 2022. PMID: 35171354 Free PMC article.
-
Long-Term Survival after Curative Resection for Postoperative Dissemination of Pancreatic Ductal Adenocarcinoma: A Case Report.Surg Case Rep. 2025;11(1):24-0022. doi: 10.70352/scrj.cr.24-0022. Epub 2025 Apr 2. Surg Case Rep. 2025. PMID: 40196210 Free PMC article.
-
Conversion surgery for initially unresectable locally advanced pancreatic ductal adenocarcinoma after chemotherapy followed by carbon-ion radiotherapy: a case report.J Med Case Rep. 2024 Jan 11;18(1):13. doi: 10.1186/s13256-023-04311-3. J Med Case Rep. 2024. PMID: 38200536 Free PMC article.
-
Resection of rectal metastasis after previous radical surgery for pancreatic cancer: Case report and literature review.Medicine (Baltimore). 2023 Dec 8;102(49):e36365. doi: 10.1097/MD.0000000000036365. Medicine (Baltimore). 2023. PMID: 38065910 Free PMC article. Review.
-
Simultaneous brain and lung metastases of pancreatic ductal adenocarcinoma after curative pancreatectomy: a case report and literature review.BMC Gastroenterol. 2021 Jan 6;21(1):9. doi: 10.1186/s12876-020-01587-3. BMC Gastroenterol. 2021. PMID: 33407200 Free PMC article. Review.
Cited by
-
Isolated abdominal wall recurrence of pancreatic ductal adenocarcinoma: a rare case report.J Surg Case Rep. 2024 Jun 21;2024(6):rjae418. doi: 10.1093/jscr/rjae418. eCollection 2024 Jun. J Surg Case Rep. 2024. PMID: 38912432 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

