Synergistic HNO3-H2SO4-NH3 upper tropospheric particle formation
- PMID: 35585346
- PMCID: PMC9117139
- DOI: 10.1038/s41586-022-04605-4
Synergistic HNO3-H2SO4-NH3 upper tropospheric particle formation
Abstract
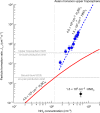
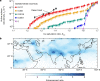
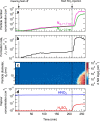
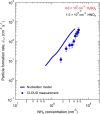
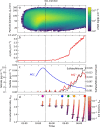
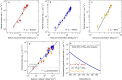
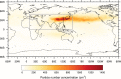
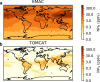
New particle formation in the upper free troposphere is a major global source of cloud condensation nuclei (CCN)1-4. However, the precursor vapours that drive the process are not well understood. With experiments performed under upper tropospheric conditions in the CERN CLOUD chamber, we show that nitric acid, sulfuric acid and ammonia form particles synergistically, at rates that are orders of magnitude faster than those from any two of the three components. The importance of this mechanism depends on the availability of ammonia, which was previously thought to be efficiently scavenged by cloud droplets during convection. However, surprisingly high concentrations of ammonia and ammonium nitrate have recently been observed in the upper troposphere over the Asian monsoon region5,6. Once particles have formed, co-condensation of ammonia and abundant nitric acid alone is sufficient to drive rapid growth to CCN sizes with only trace sulfate. Moreover, our measurements show that these CCN are also highly efficient ice nucleating particles-comparable to desert dust. Our model simulations confirm that ammonia is efficiently convected aloft during the Asian monsoon, driving rapid, multi-acid HNO3-H2SO4-NH3 nucleation in the upper troposphere and producing ice nucleating particles that spread across the mid-latitude Northern Hemisphere.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Clarke A, et al. Nucleation in the equatorial free troposphere: favorable environments during PEM-Tropics. J. Geophys. Res. Atmos. 1999;104:5735–5744. doi: 10.1029/98JD02303. - DOI
-
- Weigel R, et al. In situ observations of new particle formation in the tropical upper troposphere: the role of clouds and the nucleation mechanism. Atmos. Chem. Phys. 2011;11:9983–10010. doi: 10.5194/acp-11-9983-2011. - DOI
-
- Gordon H, et al. Causes and importance of new particle formation in the present-day and pre-industrial atmospheres. J. Geophys. Res. Atmos. 2017;122:8739–8760. doi: 10.1002/2017JD026844. - DOI
-
- Höpfner M, et al. First detection of ammonia (NH3) in the Asian summer monsoon upper troposphere. Atmos. Chem. Phys. 2016;16:14357–14369. doi: 10.5194/acp-16-14357-2016. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

