Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: the ATLAS Study
- PMID: 35585374
- PMCID: PMC9587202
- DOI: 10.1007/s13311-022-01237-4
Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: the ATLAS Study
Erratum in
-
Correction to: Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: the ATLAS Study.Neurotherapeutics. 2022 Sep;19(5):1686. doi: 10.1007/s13311-022-01286-9. Neurotherapeutics. 2022. PMID: 36175782 Free PMC article. No abstract available.
Abstract
Despite extensive research, amyotrophic lateral sclerosis (ALS) remains a progressive and invariably fatal neurodegenerative disease. Limited knowledge of the underlying causes of ALS has made it difficult to target upstream biological mechanisms of disease, and therapeutic interventions are usually administered relatively late in the course of disease. Genetic forms of ALS offer a unique opportunity for therapeutic development, as genetic associations may reveal potential insights into disease etiology. Genetic ALS may also be amenable to investigating earlier intervention given the possibility of identifying clinically presymptomatic, at-risk individuals with causative genetic variants. There is increasing evidence for a presymptomatic phase of ALS, with biomarker data from the Pre-Symptomatic Familial ALS (Pre-fALS) study showing that an elevation in blood neurofilament light chain (NfL) precedes phenoconversion to clinically manifest disease. Tofersen is an investigational antisense oligonucleotide designed to reduce synthesis of superoxide dismutase 1 (SOD1) protein through degradation of SOD1 mRNA. Informed by Pre-fALS and the tofersen clinical development program, the ATLAS study (NCT04856982) is designed to evaluate the impact of initiating tofersen in presymptomatic carriers of SOD1 variants associated with high or complete penetrance and rapid disease progression who also have biomarker evidence of disease activity (elevated plasma NfL). The ATLAS study will investigate whether tofersen can delay the emergence of clinically manifest ALS. To our knowledge, ATLAS is the first interventional trial in presymptomatic ALS and has the potential to yield important insights into the design and conduct of presymptomatic trials, identification, and monitoring of at-risk individuals, and future treatment paradigms in ALS.
Keywords: Genetic testing; Neurofilament; Phenoconversion; Pre-fALS; SOD1-ALS.
© 2022. The Author(s).
Figures

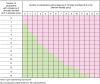

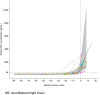


Comment in
-
Preventing Amyotrophic Lateral Sclerosis Before It Starts: When to Intervene?Neurotherapeutics. 2022 Jul;19(4):1246-1247. doi: 10.1007/s13311-022-01272-1. Epub 2022 Aug 1. Neurotherapeutics. 2022. PMID: 35913611 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

