Reproxalap Activity and Estimation of Clinically Relevant Thresholds for Ocular Itching and Redness in a Randomized Allergic Conjunctivitis Field Trial
- PMID: 35585427
- PMCID: PMC9253207
- DOI: 10.1007/s40123-022-00520-z
Reproxalap Activity and Estimation of Clinically Relevant Thresholds for Ocular Itching and Redness in a Randomized Allergic Conjunctivitis Field Trial
Abstract
Introduction: This clinical trial assessed the activity of reproxalap, a novel reactive aldehyde species modulator, and estimated clinically relevant thresholds for changes in ocular itching and redness in an allergic conjunctivitis field trial.
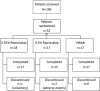
Methods: This was a randomized, double-masked, vehicle-controlled phase 2 trial. Patients with ragweed-associated allergic conjunctivitis were assessed over 28 days in an environmental setting with approximately four doses per day of either 0.25% reproxalap, 0.5% reproxalap, or vehicle. Patients recorded ocular itching, redness, tearing, and eyelid swelling scores (each with a 0-4 scale, except for a 0-3 scale for swelling), and completed the Allergic Conjunctivitis Quality of Life Questionnaire at the beginning and end of the trial.
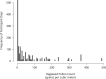
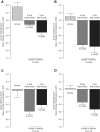
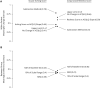
Results: Mixed model of repeated measures analysis demonstrated statistically lower itching and tearing scores (pooled P = 0.026 and P < 0.001, respectively) and numerically lower redness and eyelid swelling scores than vehicle on days when pollen exceeded the 95th percentile value. Using three anchor-based and three distribution-based approaches, the meaningful within-patient change and the between-group meaningful difference for patient-reported ocular itching and redness was estimated to be approximately 0.5. The most common treatment-emergent adverse event associated with reproxalap was transient irritation upon instillation.
Conclusion: In a field clinical trial, reproxalap was well tolerated and superior to vehicle in reducing ocular itching on high-pollen days. The clinical meaningfulness threshold estimates of 0.5 units are among the first such calculations generated for the standard ocular itching and redness scores, providing important context for the clinical interpretation of clinical trials in allergic conjunctivitis.
Keywords: Allergic conjunctivitis; Clinical relevance; Clinical trial; Ocular itching; Ocular redness; RASP modulator; Reproxalap.
Plain language summary
While allergic conjunctivitis affects millions of patients worldwide, treatments with new mechanisms have not been introduced in decades. Reproxalap, a medicine being investigated as a treatment for allergic conjunctivitis, works by regulating reactive aldehyde species—molecules that are increased in a variety of inflammatory diseases. This clinical trial assessed the activity of reproxalap and estimated what amount of change in ocular itching and redness should be considered clinically important. Patients with ragweed-associated allergic conjunctivitis were assessed over 28 days and were given one of three possible eye drops at approximately four doses per day: 0.25% reproxalap; 0.5% reproxalap; or vehicle, which was composed of the same ingredients but does not contain reproxalap. Patients recorded ocular itching, redness, tearing, and eyelid swelling (all scales ranged from 0 [none] to 4 [severe] except for eyelid swelling, which ranged from 0 to 3), and completed a quality-of-life questionnaire on allergic conjunctivitis at the beginning and end of the trial. The results indicated that reproxalap was significantly better than vehicle in reducing itching and tearing scores and was better than vehicle in reducing redness and eyelid swelling scores on days when pollen counts were high. The trial also suggested that a reduction in ocular itching and redness scores of approximately 0.5 or more (scale 0–4) is likely to be clinically important. Overall, reproxalap was well tolerated and no safety concerns were noted. The most common side effect was transient ocular discomfort after eye drop administration.
© 2022. The Author(s).
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

