Genome-wide identification of sugar transporter gene family in Brassicaceae crops and an expression analysis in the radish
- PMID: 35585498
- PMCID: PMC9115943
- DOI: 10.1186/s12870-022-03629-2
Genome-wide identification of sugar transporter gene family in Brassicaceae crops and an expression analysis in the radish
Abstract
Background: Sugar not only is an important biomacromolecule that plays important roles in plant growth, development, and biotic and abiotic stress tolerance but also provides a skeleton for other macromolecules, such as proteins and nucleic acids. Sugar transporter proteins (STPs) play essential roles in plant sugar transport and ultimately affect the abovementioned life processes. However, the evolutionary dynamics of this important gene family in Brassicaceae crops are still largely unknown, and the functional differentiation of radish STP genes remains unclear.
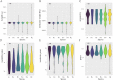
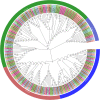
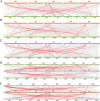
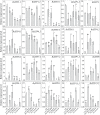
Results: In the present study, a comparative genomic study of STP genes in five representative Brassicaceae crops was conducted, and a total of 25, 25, 28, 36 and 49 STP genes were individually identified in Raphanus sativus (Rs), Brassica oleracea (Bo), B. rapa (Br), B. napus (Bn) and B. juncea (Bj), which were divided into four clades by phylogenetic analysis. The number of STP genes was no direct correlation with genome size and the total number of coding genes in Brassicaceae crops, and their physical and chemical properties showed no significant difference. Expression analysis showed that radish STP genes play vital roles not only in flower and seedpod development but also under heavy metal (cadmium, chromium and lead), NaCl and PEG-6000 stresses, Agrobacterium tumefaciens infection, and exogenous sugar treatment. RsSTP13.2 was significantly upregulated in the resistant radish cultivar by A. tumefaciens infection and induced by heavy metal, NaCl and PEG-6000 stress, indicating that it is involved in resistance to both biotic and abiotic stress in radish.
Conclusions: The present study provides insights into the evolutionary patterns of the STP gene family in Brassicaceae genomes and provides a theoretical basis for future functional analysis of STP genes in Brassicaceae crops.
Keywords: Biotic and abiotic stress; Expression analysis; Gene family; Radish; Sugar transporter proteins (STPs).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Sugar Transporter Proteins (STPs) in Gramineae Crops: Comparative Analysis, Phylogeny, Evolution, and Expression Profiling.Cells. 2019 Jun 8;8(6):560. doi: 10.3390/cells8060560. Cells. 2019. PMID: 31181814 Free PMC article.
-
Genome-wide characterization of the AP2/ERF gene family in radish (Raphanus sativus L.): Unveiling evolution and patterns in response to abiotic stresses.Gene. 2019 Nov 15;718:144048. doi: 10.1016/j.gene.2019.144048. Epub 2019 Aug 14. Gene. 2019. PMID: 31421189
-
Genome-wide identification and characterization of NBS-encoding genes in Raphanus sativus L. and their roles related to Fusarium oxysporum resistance.BMC Plant Biol. 2021 Jan 18;21(1):47. doi: 10.1186/s12870-020-02803-8. BMC Plant Biol. 2021. PMID: 33461498 Free PMC article.
-
Fungal endophytes of Brassicaceae: Molecular interactions and crop benefits.Front Plant Sci. 2022 Aug 5;13:932288. doi: 10.3389/fpls.2022.932288. eCollection 2022. Front Plant Sci. 2022. PMID: 35991403 Free PMC article. Review.
-
Molecular basis for optimizing sugar metabolism and transport during fruit development.aBIOTECH. 2021 Sep 20;2(3):330-340. doi: 10.1007/s42994-021-00061-2. eCollection 2021 Sep. aBIOTECH. 2021. PMID: 36303881 Free PMC article. Review.
References
-
- Geng Y, Dong X, Zhang C. Recent Progress of Sugar Transporters in Horticultural Crops. Acta Horticulturae Sinica. 2021;48(4):676–688.
MeSH terms
Substances
Grants and funding
- 31801858/National Natural Science Foundation of China
- jit-b-202009/Research Foundation for Talented Scholars of Jinling Institute of Technology
- jit-fhxm-202113/Research Foundation Incubation Project of Jinling Institute of Technology
- CARS-23-A09/China Agriculture Research System
- 2021C02065/New Variety Breeding Project of the Major Science Technology Projects of Zhejiang
LinkOut - more resources
Full Text Sources

