Engineered Cas9 extracellular vesicles as a novel gene editing tool
- PMID: 35585651
- PMCID: PMC9117459
- DOI: 10.1002/jev2.12225
Engineered Cas9 extracellular vesicles as a novel gene editing tool
Abstract
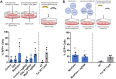
Extracellular vesicles (EVs) have shown promise as biological delivery vehicles, but therapeutic applications require efficient cargo loading. Here, we developed new methods for CRISPR/Cas9 loading into EVs through reversible heterodimerization of Cas9-fusions with EV sorting partners. Cas9-loaded EVs were collected from engineered Expi293F cells using standard methodology, characterized using nanoparticle tracking analysis, western blotting, and transmission electron microscopy and analysed for CRISPR/Cas9-mediated functional gene editing in a Cre-reporter cellular assay. Light-induced dimerization using Cryptochrome 2 combined with CD9 or a Myristoylation-Palmitoylation-Palmitoylation lipid modification resulted in efficient loading with approximately 25 Cas9 molecules per EV and high functional delivery with 51% gene editing of the Cre reporter cassette in HEK293 and 25% in HepG2 cells, respectively. This approach was also effective for targeting knock-down of the therapeutically relevant PCSK9 gene with 6% indel efficiency in HEK293. Cas9 transfer was detergent-sensitive and associated with the EV fractions after size exclusion chromatography, indicative of EV-mediated transfer. Considering the advantages of EVs over other delivery vectors we envision that this study will prove useful for a range of therapeutic applications, including CRISPR/Cas9 mediated genome editing.
Keywords: CRISPR/Cas9 delivery; exosomes; extracellular vesicles; gene editing; optogenetics.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
All authors are or were employed in AstraZeneca R&D.
Figures





References
-
- Anzalone, A. V. , Randolph, P. B. , Davis, J. R. , Sousa, A. A. , Koblan, L. W. , Levy, J. M. , Chen, P. J. , Wilson, C. , Newby, G. A. , Raguram, A. , & Liu, D. R. (2019). Search‐and‐replace genome editing without double‐strand breaks or donor DNA. Nature, 576, 149–157. 10.1038/s41586-019-1711-4 - DOI - PMC - PubMed
-
- Chen, R. , Huang, H. , Liu, H. , Xi, J. , Ning, J. , Zeng, W. , Shen, C. , Zhang, T. , Yu, G. , Xu, Q. , Chen, X. , Wang, J. , & Lu, F. (2019). Friend or foe? Evidence indicates endogenous exosomes can deliver functional gRNA and Cas9 protein. Small, 15, 1902686. 10.1002/smll.201902686 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

