SARS-CoV-2 Omicron BA.1 variant breakthrough infections in nursing home residents after an homologous third dose of the Comirnaty® COVID-19 vaccine: Looking for correlates of protection
- PMID: 35585782
- PMCID: PMC9348298
- DOI: 10.1002/jmv.27867
SARS-CoV-2 Omicron BA.1 variant breakthrough infections in nursing home residents after an homologous third dose of the Comirnaty® COVID-19 vaccine: Looking for correlates of protection
Abstract
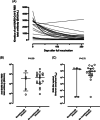
We investigated whether peripheral blood levels of SARS-CoV-2 Spike (S) receptor binding domain antibodies (anti-RBD), neutralizing antibodies (NtAb) targeting Omicron S, and S-reactive-interferon (IFN)-γ-producing CD4+ and CD8+ T cells measured after a homologous booster dose (3D) with the Comirnaty® vaccine was associated with the likelihood of subsequent breakthrough infections due to the Omicron variant. An observational study including 146 nursing home residents (median age, 80 years; range, 66-99; 109 female) evaluated for an immunological response after 3D (at a median of 16 days). Anti-RBD total antibodies were measured by chemiluminescent immunoassay. NtAb were quantified by an Omicron S pseudotyped virus neutralization assay. SARS-CoV-2-S specific-IFNγ-producing CD4+ and CD8+ T cells were enumerated by whole-blood flow cytometry for intracellular cytokine staining. In total, 33/146 participants contracted breakthrough Omicron infection (symptomatic in 30/33) within 4 months after 3D. Anti-RBD antibody levels were comparable in infected and uninfected participants (21 123 vs. 24 723 BAU/ml; p = 0.34). Likewise, NtAb titers (reciprocal IC50 titer, 157 vs. 95; p = 0.32) and frequency of virus-reactive CD4+ (p = 0.82) and CD8+ (p = 0.91) T cells were similar across participants in both groups. anti-RBD antibody levels and NtAb titers estimated at around the time of infection were also comparable (3445 vs. 4345 BAU/ml; p = 0.59 and 188.5 vs. 88.9; p = 0.70, respectively). Having detectable NtAb against Omicron or SARS-CoV-2-S-reactive-IFNγ-producing CD4+ or CD8+ T cells after 3D was not correlated with increased protection from breakthrough infection (OR, 1.50; p = 0.54; OR, 0.0; p = 0.99 and OR 3.70; p = 0.23, respectively). None of the immune parameters evaluated herein, including NtAb titers against the Omicron variant, may reliably predict at the individual level the risk of contracting COVID-19 due to the Omicron variant in nursing home residents.
Keywords: Comirnaty® COVID-19 vaccine; SARS-CoV-2 Omicron variant; anti-spike antibodies; breakthrough infection; neutralizing antibodies; nursing home residents; spike-reactive T cells.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS‐CoV‐2 Omicron to antibody neutralization. Nature. 2022;602:671‐675. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

