Effects of Sublethal Organophosphate Toxicity and Anti-cholinergics on Electroencephalogram and Respiratory Mechanics in Mice
- PMID: 35585917
- PMCID: PMC9108673
- DOI: 10.3389/fnins.2022.866899
Effects of Sublethal Organophosphate Toxicity and Anti-cholinergics on Electroencephalogram and Respiratory Mechanics in Mice
Abstract
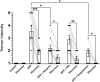
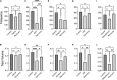
Organophosphates are used in agriculture as insecticides but are potentially toxic to humans when exposed at high concentrations. The mechanism of toxicity is through antagonism of acetylcholinesterase, which secondarily causes excess activation of cholinergic receptors leading to seizures, tremors, respiratory depression, and other physiological consequences. Here we investigated two of the major pathophysiological effects, seizures and respiratory depression, using subcutaneous injection into mice of the organophosphate diisopropylfluorophosphate (DFP) at sublethal concentrations (2.1 mg/Kg) alone and co-injected with current therapeutics atropine (50 mg/Kg) or acetylcholinesterase reactivator HI6 (3 mg/Kg). We also tested a non-specific cholinergic antagonist dequalinium chloride (2 mg/Kg) as a novel treatment for organophosphate toxicity. Electroencephalogram (EEG) recordings revealed that DFP causes focal delta frequency (average 1.4 Hz) tonic spikes in the parietal region that occur transiently (lasting an average of 171 ± 33 min) and a more sustained generalized theta frequency depression in both parietal and frontal electrode that did not recover the following 24 h. DFP also caused behavioral tremors that partially recovered the following 24 h. Using whole body plethysmography, DFP revealed acute respiratory depression, including reduced breathing rates and tidal volumes, that partially recover the following day. Among therapeutic treatments, dequalinium chloride had the most potent effect on all physiological parameters by reducing acute EEG abnormalities and promoting a full recovery after 24 h from tremors and respiratory depression. Atropine and HI6 had distinct effects on EEGs. Co-treatment with atropine converted the acute 1.4 Hz tonic spikes to 3 Hz tonic spikes in the parietal electrode and promoted a partial recovery after 24 h from theta frequency and respiratory depression. HI6 fully removed the parietal delta spike increase and promoted a full recovery in theta frequency and respiratory depression. In summary, while all anticholinergic treatments promoted survival and moderated symptoms of DFP toxicity, the non-selective anti-cholinergic dequalinium chloride had the most potent therapeutic effects in reducing EEG abnormalities, moderating tremors and reducing respiratory depression.
Keywords: EEG; anticholinergic; organophosphate; respiration; seizure.
Copyright © 2022 Bugay, Gregory, Belanger-Coast, Zhao and Brenner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Pretreatment with pyridostigmine bromide has no effect on seizure behavior or 24 hour survival in the rat model of acute diisopropylfluorophosphate intoxication.Neurotoxicology. 2019 Jul;73:81-84. doi: 10.1016/j.neuro.2019.03.001. Epub 2019 Mar 7. Neurotoxicology. 2019. PMID: 30853371 Free PMC article.
-
Organophosphate-induced convulsions and prevention of neuropathological damages.Toxicology. 2004 Mar 1;196(1-2):31-9. doi: 10.1016/j.tox.2003.10.013. Toxicology. 2004. PMID: 15036754
-
Increased diisopropylfluorophosphate-induced toxicity in mu-opioid receptor knockout mice.J Neurosci Res. 2004 Oct 15;78(2):259-67. doi: 10.1002/jnr.20259. J Neurosci Res. 2004. PMID: 15378609
-
Management of acute childhood poisonings caused by selected insecticides and herbicides.Pediatr Clin North Am. 1986 Apr;33(2):421-45. doi: 10.1016/s0031-3955(16)35012-x. Pediatr Clin North Am. 1986. PMID: 3515303 Review.
-
Unequal efficacy of pyridinium oximes in acute organophosphate poisoning.Clin Med Res. 2007 Mar;5(1):71-82. doi: 10.3121/cmr.2007.701. Clin Med Res. 2007. PMID: 17456837 Free PMC article. Review.
Cited by
-
Modifiable contributing factors to COVID-19: A comprehensive review.Food Chem Toxicol. 2023 Jan;171:113511. doi: 10.1016/j.fct.2022.113511. Epub 2022 Nov 28. Food Chem Toxicol. 2023. PMID: 36450305 Free PMC article. Review.
-
Cardiovascular responses of adult male Sprague-Dawley rats following acute organophosphate intoxication and post-exposure treatment with midazolam with or without allopregnanolone.Arch Toxicol. 2024 Apr;98(4):1177-1189. doi: 10.1007/s00204-023-03679-x. Epub 2024 Feb 2. Arch Toxicol. 2024. PMID: 38305864 Free PMC article.
-
Acute Treatment with the M-Channel (Kv7, KCNQ) Opener Retigabine Reduces the Long-Term Effects of Repetitive Blast Traumatic Brain Injuries.Neurotherapeutics. 2023 Apr;20(3):853-869. doi: 10.1007/s13311-023-01361-9. Epub 2023 Mar 28. Neurotherapeutics. 2023. PMID: 36976493 Free PMC article.
References
LinkOut - more resources
Full Text Sources

