Dietary Supplementation With Acer truncatum Oil Promotes Remyelination in a Mouse Model of Multiple Sclerosis
- PMID: 35585921
- PMCID: PMC9109879
- DOI: 10.3389/fnins.2022.860280
Dietary Supplementation With Acer truncatum Oil Promotes Remyelination in a Mouse Model of Multiple Sclerosis
Abstract
Background: Multiple sclerosis is a chronic demyelinating disease of uncertain etiology. Traditional treatment methods produce more adverse effects. Epidemiological and clinical treatment findings showed that unknown environmental factors contribute to the etiology of MS and that diet is a commonly assumed factor. Despite the huge interest in diet expressed by people with MS and the potential role diet plays in MS, very little data is available on the role of diet in MS pathogenesis and MS course, in particular, studies on fats and MS. The oil of Acer truncatum is potential as a resource to be exploited in the treatment of some neurodegenerative diseases.
Objective: Here, we investigated the underlying influences of Acer truncatum oil on the stimulation of remyelination in a cuprizone mouse model of demyelination.
Methods: Cuprizone (0.2% in chow) was used to establish a mouse model of demyelination. Acer truncatum oil was administrated to mice during remyelination. Following techniques were used: behavioral test, histochemistry, fluorescent immunohistochemistry, transmission electron microscope.
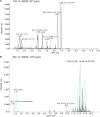
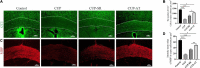
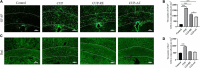
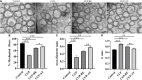
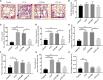
Results: Mice exposed to cuprizone for 6 weeks showed schizophrenia-like behavioral changes, the increased exploration of the center in the open field test (OFT), increased entries into the open arms of the elevated plus-maze, as well as demyelination in the corpus callosum. After cuprizone withdrawal, the diet therapy was initiated with supplementation of Acer truncatum oil for 2 weeks. As expected, myelin repair was greatly enhanced in the demyelinated regions with increased mature oligodendrocytes (CC1) and myelin basic protein (MBP). More importantly, the supplementation with Acer truncatum oil in the diet reduced the schizophrenia-like behavior in the open field test (OFT) and the elevated plus-maze compared to the cuprizone recovery group. The results revealed that the diet supplementation with Acer truncatum oil improved behavioral abnormalities, oligodendrocyte maturation, and remyelination in the cuprizone model during recovery.
Conclusion: Diet supplementation with Acer truncatum oil attenuates demyelination induced by cuprizone, indicating that Acer truncatum oil is a novel therapeutic diet in demyelinating diseases.
Keywords: Acer truncatum oil; cuprizone; demyelination; neurodegenerative disease; remyelination.
Copyright © 2022 Xue, Zhu, Yan, Zhang, Cui, Wu, Li, Pan, Yan, Chai and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


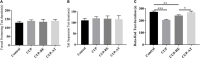
Similar articles
-
Beneficial Effects of Plant Oils Supplementation on Multiple Sclerosis: A Comprehensive Review of Clinical and Experimental Studies.Nutrients. 2023 Nov 18;15(22):4827. doi: 10.3390/nu15224827. Nutrients. 2023. PMID: 38004221 Free PMC article. Review.
-
Clemastine rescues behavioral changes and enhances remyelination in the cuprizone mouse model of demyelination.Neurosci Bull. 2015 Oct;31(5):617-25. doi: 10.1007/s12264-015-1555-3. Epub 2015 Aug 6. Neurosci Bull. 2015. PMID: 26253956 Free PMC article.
-
rHIgM22 enhances remyelination in the brain of the cuprizone mouse model of demyelination.Neurobiol Dis. 2017 Sep;105:142-155. doi: 10.1016/j.nbd.2017.05.015. Epub 2017 May 30. Neurobiol Dis. 2017. PMID: 28576706
-
Effects of Bone Marrow Mesenchymal Stem Cells on Myelin Repair and Emotional Changes of a Cuprizone-Induced Demyelination Model.J Integr Neurosci. 2023 Feb 16;22(2):40. doi: 10.31083/j.jin2202040. J Integr Neurosci. 2023. PMID: 36992584
-
The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system.Brain Pathol. 2001 Jan;11(1):107-16. doi: 10.1111/j.1750-3639.2001.tb00385.x. Brain Pathol. 2001. PMID: 11145196 Free PMC article. Review.
Cited by
-
Beneficial Effects of Plant Oils Supplementation on Multiple Sclerosis: A Comprehensive Review of Clinical and Experimental Studies.Nutrients. 2023 Nov 18;15(22):4827. doi: 10.3390/nu15224827. Nutrients. 2023. PMID: 38004221 Free PMC article. Review.
-
Remyelination in Multiple Sclerosis: Findings in the Cuprizone Model.Int J Mol Sci. 2022 Dec 17;23(24):16093. doi: 10.3390/ijms232416093. Int J Mol Sci. 2022. PMID: 36555733 Free PMC article. Review.
-
Multi-omics reveal neuroprotection of Acer truncatum Bunge Seed extract on hypoxic-ischemia encephalopathy rats under high-altitude.Commun Biol. 2023 Oct 2;6(1):1001. doi: 10.1038/s42003-023-05341-9. Commun Biol. 2023. PMID: 37783835 Free PMC article.
References
-
- Alter M., Yamoor M., Harshe M. (1974). Multiple sclerosis and nutrition. Arch. Neurol. 31, 267–72. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

