Lysine Acetylation/Deacetylation Modification of Immune-Related Molecules in Cancer Immunotherapy
- PMID: 35585975
- PMCID: PMC9108232
- DOI: 10.3389/fimmu.2022.865975
Lysine Acetylation/Deacetylation Modification of Immune-Related Molecules in Cancer Immunotherapy
Abstract
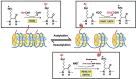
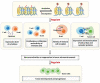
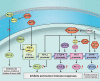
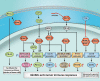
As major post-translational modifications (PTMs), acetylation and deacetylation are significant factors in signal transmission and cellular metabolism, and are modulated by a dynamic process via two pivotal categories of enzymes, histone acetyltransferases (HATs) and histone deacetylases (HDACs). In previous studies, dysregulation of lysine acetylation and deacetylation has been reported to be associated with the genesis and development of malignancy. Scientists have recently explored acetylation/deacetylation patterns and prospective cancer therapy techniques, and the FDA has approved four HDAC inhibitors (HDACi) to be used in clinical treatment. In the present review, the most recent developments in the area of lysine acetylation/deacetylation alteration in cancer immunotherapy were investigated. Firstly, a brief explanation of the acetylation/deacetylation process and relevant indispensable enzymes that participate therein is provided. Subsequently, a multitude of specific immune-related molecules involved in the lysine acetylation/deacetylation process are listed in the context of cancer, in addition to several therapeutic strategies associated with lysine acetylation/deacetylation modification in cancer immunotherapy. Finally, a number of prospective research fields related to cancer immunotherapy concepts are offered with detailed analysis. Overall, the present review may provide a reference for researchers in the relevant field of study, with the aim of being instructive and meaningful to further research as well as the selection of potential targets and effective measures for future cancer immunotherapy strategies.
Keywords: Cancer; HAT; HDAC; acetylation; deacetylation; immunotherapy.
Copyright © 2022 Ding, Ma, Liu, Pan, Li, Feng, Zhang, Shao, Jiang, Lu, Han, Wang and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
HAT- and HDAC-Targeted Protein Acetylation in the Occurrence and Treatment of Epilepsy.Biomedicines. 2022 Dec 29;11(1):88. doi: 10.3390/biomedicines11010088. Biomedicines. 2022. PMID: 36672596 Free PMC article. Review.
-
Deacetylation of nonhistone proteins by HDACs and the implications in cancer.Handb Exp Pharmacol. 2011;206:39-56. doi: 10.1007/978-3-642-21631-2_3. Handb Exp Pharmacol. 2011. PMID: 21879445 Review.
-
Histone Acetylation Enzymes Coordinate Metabolism and Gene Expression.Trends Plant Sci. 2015 Oct;20(10):614-621. doi: 10.1016/j.tplants.2015.07.005. Trends Plant Sci. 2015. PMID: 26440431 Review.
-
Nonhistone protein acetylation as cancer therapy targets.Expert Rev Anticancer Ther. 2010 Jun;10(6):935-54. doi: 10.1586/era.10.62. Expert Rev Anticancer Ther. 2010. PMID: 20553216 Free PMC article. Review.
-
Comprehensive analysis for histone acetylation of human colon cancer cells treated with a novel HDAC inhibitor.Curr Pharm Des. 2014;20(11):1866-73. doi: 10.2174/13816128113199990531. Curr Pharm Des. 2014. PMID: 23888955
Cited by
-
Histone deacetylases: Regulation of vascular homeostasis via endothelial cells and vascular smooth muscle cells and the role in vascular pathogenesis.Genes Dis. 2024 Jan 22;11(6):101216. doi: 10.1016/j.gendis.2024.101216. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39281836 Free PMC article. Review.
-
Targeting epigenetic regulators to overcome drug resistance in cancers.Signal Transduct Target Ther. 2023 Feb 17;8(1):69. doi: 10.1038/s41392-023-01341-7. Signal Transduct Target Ther. 2023. PMID: 36797239 Free PMC article. Review.
-
Emerging Neuroprotective Strategies: Unraveling the Potential of HDAC Inhibitors in Traumatic Brain Injury Management.Curr Neuropharmacol. 2024;22(14):2298-2313. doi: 10.2174/1570159X22666240128002056. Curr Neuropharmacol. 2024. PMID: 38288835 Free PMC article. Review.
-
Histone deacetylases 10 as a prognostic biomarker correlates with tumor microenvironment and therapy response in colorectal cancer.World J Gastroenterol. 2025 Jul 14;31(26):108662. doi: 10.3748/wjg.v31.i26.108662. World J Gastroenterol. 2025. PMID: 40678705 Free PMC article.
-
Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets.MedComm (2020). 2023 May 20;4(3):e292. doi: 10.1002/mco2.292. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37220590 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

